1. Background
Hepatitis C virus (HCV) is a blood-borne, positive sense, single-stranded ribonucleic acid (RNA) virus of the family Flaviviridae. HCV can cause mild to severe liver diseases and is the most common cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC). HCV prevalence ranges 0.2% to 40% in different countries, affecting over 170 - 200 million people worldwide. Chronic infection with hepatitis C and related diseases are global health problems and impose heavy financial burden on the health systems and societies (1).
Based on genetic differences, the HCV species are classified into 6 genotypes (1 to 6) with several subtypes within each genotype (2, 3). Genotype 1 (GT1) is the most common genotype of HCV infection, accounting for 46% - 60% of cases worldwide (4, 5). The subtypes genotype 1a (GT1a) and genotype 1b (GT1b) are responsible for the vast majority of GT1 infections (6).
In patients with HCV GT1 infection the rates of sustained virologic response (SVR12) after treatment with pegylated interferon and ribavirin (PegINF/RBV) were as low as 50% and 30% in non-transplant and liver transplant recipients (6-8).
SVR12 rates increased approximately up to 60% - 80% in HCV GT1-infected non-transplant patients (9, 10) and up to 52% in liver transplant recipients since 2011, when a new family of medicines known as first generation direct-acting antivirals (DAAs) telaprevir and boceprevir were developed and introduced for use in combination with PegINF/RBV (8). PegINF, however, is associated with substantial side effects, which affect adherence to the interferon-based therapy (11).
The new interferon-free second-generation DAA therapy (3D therapy) consisting of ombitasvir (OBV)/paritaprevir (PTV)/ritonavir (r) ± dasabuvir (DSV) ± ribavirin (RBV) improved the efficacy, safety, and tolerability of the treatment of chronic HCV infection. In clinical studies, the regimen consisting of OBV/PTV/r + DSV ± RBV was highly efficacious to treat HCV GT1a or GT1b infection, including patients with compensated cirrhosis, liver transplant or human immunodeficiency virus 1 (HIV-1) co-infection. The observed SVR12 rate ranged from 92% to 100% (8, 12-19).
The combination of the NS5A inhibitor ombitasvir, the NS3/4A protease inhibitor paritaprevir boosted with ritonavir, and the NS5B polymerase inhibitor dasabuvir with or without ribavirin was approved to treat chronic HCV GT1 infection in the United Stated in December 2014 and in European Union in January 2015 (17, 20).
The current multicentre open-label, uncontrolled, real-world study aimed at evaluating the efficacy and safety of the OBV/PTV/r + DSV ± RBV in the early access program in Lithuania and Latvia and in clinical practice in Lithuania from January 2015 to November 2016.
2. Methods
2.1. Study Population
Adult patients with chronic HCV (CHC) infection treated with OBV/PTV/r + DSV ± RBV at Vilnius university hospital Santaros Klinikos centre of hepatology, gastroenterology and dietetics, centre of infectious diseases (Vilnius, Lithuania) and Riga East university hospital, infectology centre of Latvia, hepatology department (Riga, Latvia) from January 2015 to November 2016 were enrolled into the current study. Patients were eligible for OBV/PTV/r + DSV ± RBV treatment if infected with HCV GT1a, 1b, or 1a/b, were treatment-naïve or previously treated with PegINF/RBV ± first generation protease inhibitor telaprevir or boceprevir, and/or had advanced fibrosis (METAVIR stage F3 - F4). Eligible patients also included the ones with post-orthotopic liver transplantation and patients with F2 fibrosis stage with contraindications to interferon-based therapy or not tolerate to PegINF/RBV regimen. Patients with advanced liver decompensation and Child-Pugh C cirrhosis were excluded from the study.
2.2. Study Design
It was an international multicentre open-label, uncontrolled, real-world retrospective study conducted in Lithuania (2 centres) and Latvia (1 centre). The local hospitals ethics committees in each country approved the study.
The treatment of the OBV/PTV/r + DSV ± RBV for the part of patients was obtained within an early access patient program from January 2015 and written informed consent was obtained from each participitant. The therapy for the remaining patients was 100% reimbursed in October 29, 2015 in Lithuania and January 1, 2016 in Latvia.
Clinical assessments, concomitant treatments and other medical decisions were taken at the discretion of the treating physicians according to standard clinical practice. In accordance to the approved summaries of product characteristics, patients received a combination of OBV/PTV/r (25 mg, 150 mg, and 100 mg daily) and DSV (250 mg daily) with or without weight-based RBV (1000 - 1200 mg daily) for 12 or 24 weeks. Patients infected with GT1a were treated with OBV/PTV/r + DSV + RBV for 12 weeks (the ones without cirrhosis) or for 24 weeks (the ones with compensated cirrhosis and all patients undergone liver transplantation). All patients with GT1b-infection were treated with OBV/PTV/r + DSV ± RBV for 12 weeks. The dose of RBV was adjusted during the course of treatment according to laboratory test results and tolerability. In liver transplant recipients, the doses of immunosuppressive agents (tacrolimus and cyclosporine) were reduced according to plasma concentrations.
Patients data were collected retrospectively by reviewing individual’s health record. Baseline demographic and clinical data included gender, age, body mass index (BMI), fibrosis stage (METAVIR scoring system), HCV genotype, previous antiviral treatment, and response to previous treatments. Fibrosis was evaluated by liver biopsy and/or transient elastography (FibroScan). The Child-Pugh score system was used to define clinical status.
Laboratory parameters and adverse events (AE) were assessed at the baseline (the day 0), the weeks 1, 2, 4, 8, at the end of treatment (EOT, week 12 or 24), and at the follow-up (FU, 12 weeks after EOT), or until the premature discontinuation of treatment. Laboratory data included HCV ribonucleic acid (RNA) level, alpha-fetoprotein (AFP), international normalised ratio (INR), albumins, bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), serum creatinine, platelets count (PLT), haemoglobin (Hb). HCV-RNA level was measured using real-time polymerase chain reaction (real-time PCR) according to standard methods at the baseline, at the EOT, and at FU visit.
At each visit, patients were interviewed to identify any adverse event.
2.3. Virologic and Safety Assessment
The primary effectiveness endpoint was SVR12, following the end of treatment. The secondary endpoints included end-of-treatment response (undetectable HCV RNA at the end of treatment), virologic relapse (undetectable HCV RNA at the end of treatment, but positive within 12 weeks post treatment), and non-response (HCV RNA being detectable at the end of treatment).
Safety endpoints included adverse events (AE) and laboratory abnormalities.
2.4. Serological and Molecular Diagnosis of Viral Spread
In the first step, to evaluate the exposure rate the presence of total anti-HCV antibodies was determined using 4th generation anti-HCV enzyme-linked immunosorbent assay (ELISA) kit on serum samples according to the manufacturer’s instructions.
Then, to investigate the current molecular state in cases with positive HCV antibody, viral RNA genome was extracted from sera by a commercially available kit. In addition, for more accurate evaluation, a commercial real-time PCR detection method was employed to confirm the presence of viral genome inside the serum. Finally, a simple genotyping assay was performed on family members with PCR positive results (21).
2.5. Statistical Analysis
The descriptive statistics and statistical tests for group comparisons were applied for data analysis. Means of 2 dependent samples were compared using the paired samples t test. For categorical variables, differences between the 2 groups were evaluated using the Pearson chi-square test. Statistical differences were interpreted at 5% (2-sided) significance level. Statistical analyses were performed using IBM SPSS 20.0 and Microsoft Excel 2010 software.
3. Results
3.1. Patients disposition
The current study participants consisted of 134 patients with HCV GT-1 infection, including 10 liver transplant recipients. Patients demographics and baseline clinical characteristics are presented in Table 1. Among non-transplant patients, the most prevalent HCV subtype was 1b (88.7%). Majority of non-transplant patients (72.6%) were previously treated with PegIFN/RBV, 26 (21.0%) patients received triple therapy with PegIFN/RBV, and telaprevir or boceprevir. All except one liver transplant recipient were infected with HCV GT-1b, and 8 (20.0%) patients were treatment-experienced.
| Characteristics | Non-Transplant Patient, N = 124 | Liver Transplant Recipient, N = 10 |
|---|---|---|
| Gender, n (%) | ||
| Male | 62 (50.0%) | 4 (40.0%) |
| Female | 62 (50.0%) | 6 (60.0%) |
| Age, year | ||
| Mean ± SD | 54.6 ± 11 | 50.7 ± 8.0 |
| Range (min - max) | 24 - 83 | 42 - 63 |
| BMI, kg/m2 | ||
| Mean ± SD | 28.1 ± 4.9 | 25.6 ± 4.2 |
| Range (min - max) | 18 - 42 | 20 - 31 |
| Genotype | ||
| 1a | 7 (5.6%) | 0 |
| 1b | 110 (88.7%) | 9 (90.0%) |
| 1a/b | 7 (5.6%) | 1 (10.0%) |
| Fibrosis stage | ||
| F1 | 0 | 2 (20.0%) |
| F2 | 10 (8.1%) | 3 (30.0%) |
| F3 | 43 (34.7%) | 1 (10.0%) |
| F4 | 71 (57.3%) | 0 |
| Unknown | 0 | 4 (40.0%) |
| HCV-RNA, IU/mL | ||
| Mean ± SD | 5.0 × 106 ± 9.5 × 106 | 8.7 × 106 ± 1.4 × 107 |
| Range (min - max) | 8.7 × 103 - 8.8 × 107 | 1.6 × 103 - 5.1 × 107 |
| Antiviral treatment history | ||
| Naive | 34 (27.4%) | 2 (20.0%) |
| Triple therapy with boceprevir or telaprevir | 26 (21.0%) | 1 (10.0%) |
| Null-responders | 36 (29.0%) | 4 (40.0%) |
| Partial responders | 18 (14.5%) | 1 (10.0%) |
| Relapsers | 27 (21.8%) | 2 (20.0%) |
| Discontinued due to AE | 9 (7.8%) | 1 (10.0%) |
Abbreviations: AE, adverse event; BMI, body mass index; HCV, hepatitis C virus; RNA, ribonucleic acid; SD, standard deviation.
A total of 120 non-transplant patients received a 12-week treatment, while all 10 liver transplant recipients and 4 GT-1a infected non-transplant patients with compensated cirrhosis were treated for 24 weeks; 104 patients also received RBV. The scheduled treatment was completed for 121 (97.4%) non-transplant patients and all 10 (100.0%) liver transplanted recipients. Three non-transplant patients prematurely discontinued the treatment due to AEs.
3.2. Effectiveness
HCV RNA was undetectable at the EOT in 122 (98.4%) non-transplant patients. SVR12 was achieved in 120 (96.8%) non-transplant patients. SVR12 rates were high across different subgroups (Table 2). All liver transplant recipients (100%) achieved virological suppression at EOT and 12 weeks after the treatment (SVR12).
| Patients Group | VR at EOT | SVR12 |
|---|---|---|
| Non-transplant patients | 122/124 (98.4) | 120/124 (96.8) |
| Cirrhotic patients | 70/72 (97.2) | 69/72 (95.8) |
| Liver transplant recipients | 10/10 (100.0) | 10/10 (100.0) |
| Non-transplant patients according to treatment history | ||
| Naive | 34/35 (97.1) | 34/35 (97.1) |
| Null-responders to PegIFN/RBV + telaprevir or Boceprevir | 7/8 (87.5) | 7/8 (87.5) |
| Null-responders to PegIFN/RBV | 31/31 (100.0) | 30/31 (96.8) |
| Partial responders | 18/18 (100.0) | 18/18 (100.0) |
| Relapsers | 27/27 (100.0) | 26/27 (96.3) |
Abbreviations: EOT, end of treatment; PegIFN, pegylated interferon; RBV, ribavirin; VR, virologic response; SVR12, sustained virologic response at 12 weeks post-treatment.
Among 4 treatment failures, 2 patients were non-responders and 2 patients relapsed. Three patients also failed their previous antiviral treatment. Characteristics of patients with treatment failures are summarized in Table 3.
| Patient No. 1 | Patient No. 2 | Patient No. 3 | Patient No. 4 | |
|---|---|---|---|---|
| Gender | Male | Male | Male | Male |
| Age, years | 43 | 53 | 38 | 45 |
| Fibrosis stage | F4 | F4 | F3 | F4 |
| Genotype | 1a/b | 1b | 1b | 1b |
| Previous antiviral treatment | PegINF/RBV+, Boceprevir -, Null-responder | PegINF/RBV -, null-responder | PegINF/RBV+, Boceprevir -, relapser | Treatment-naive |
| Scheduled duration of treatment with OBV/PTV/r + DSV ± RBV | 24 weeks | 12 weeks | 12 weeks | 12 weeks |
| RBV | 1200 mg (RBV dose was not reduced) | 1200 mg (RBV dose was not reduced) | 1200 mg (RBV dose was not reduced) | 1200 mg (RBV dose was not reduced) |
| HCV-RNA, IU/mL | ||||
| Day 0 | 3490000 | 2610000 | 142500 | 5790000 |
| EOT | 374000 | Undetected | Undetected | - |
| FU | - | 3210000 | 1750000 | - |
| Adverse events | Fatigue Bleeding from the nose | - | Asthenia, Fatigue, Insomnia, ALT↑5xULN, (273 µM/L at the week 4) | Skin itching, Rash, (psoriasis exacerbation) |
Abbreviations: ALT, alanine transaminase; DSV, dasabuvir; HCV, hepatitis C virus; OBV, ombitasvir; PegINF, pegylated interferon; PTV, paritaprevir; r, ritonavir; RNA, ribonucleic acid; RBV, ribavirin; ULN, upper limit of normal.
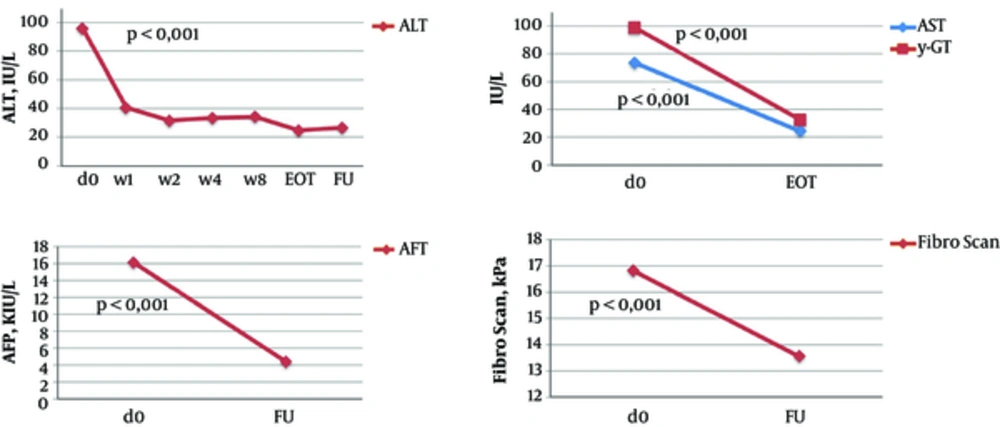
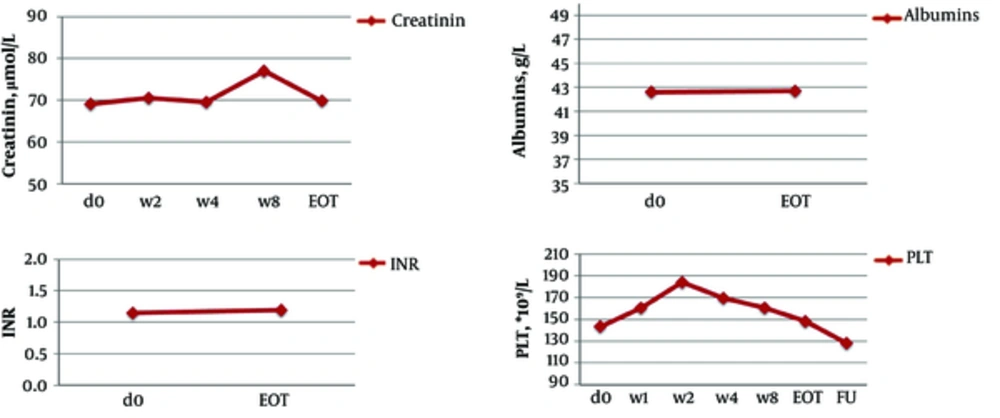
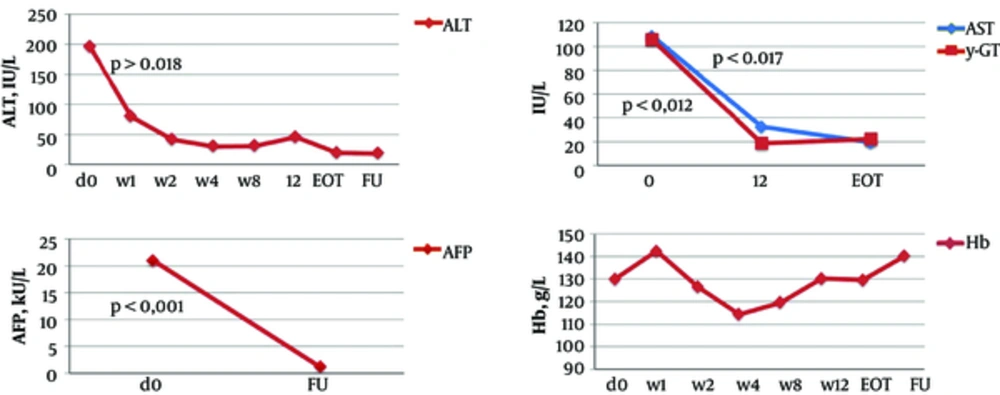
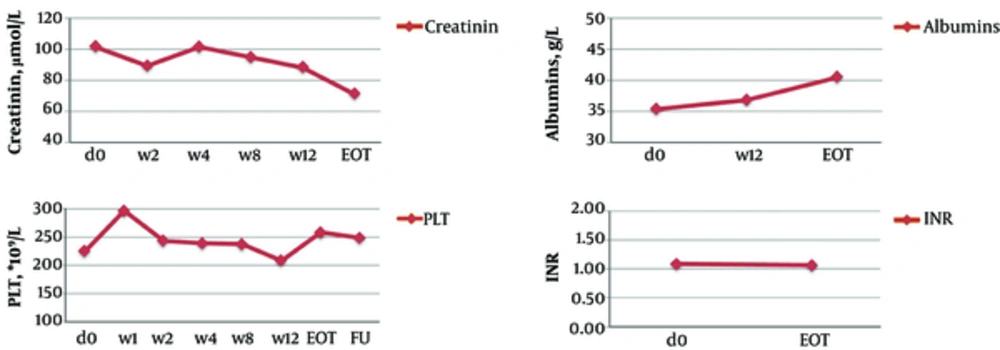
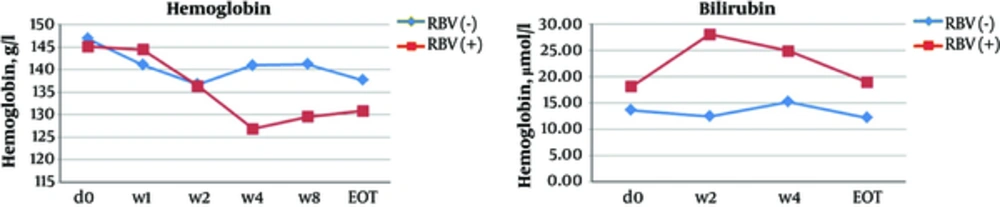
In non-transplant patients, the mean ALT level significantly decreased from 95.7 IU/L at baseline to 40.7 IU/L after the first week of the treatment (P ≤ 0.001) and to 26.7 IU/L at the EOT (P ≤ 0.001). AST, γ-GT, and AFP levels and liver stiffness also significantly improved at the EOT as compared to baseline (Table 4, Figures 1 - 2). At the EOT, ALT, AST, and γ-GT levels were normalised in89.0%, 95.3%, and 67.6% of patients with elevated baseline levels, respectively. Similarly, significant improvement in liver function tests was observed in liver transplant recipients (Table 4, Figures 3 - 4).
| Non-Transplanted Patients, n = 124 | Liver Transplant Patients, n = 10 | |||||
|---|---|---|---|---|---|---|
| D0 | EOT | FU | D0 | EOT | FU | |
| FibroScan, kPa | NA | NA | NA | |||
| Mean | 16.9 ± 11.1 | 13.5 ± 8.2a | 13.7 ± 8.6 | |||
| Range (min-max) | 3.5 - 69.7 | 3.6 - 45.7 | 3.3 - 45.7 | |||
| Alpha-fetoprotein (AFP), kIU/L | NA | |||||
| Mean | 16.1 ± 26.5 | NA | 4.3 ± 2.9a | 21.0 ± 32.5 | 1.2 ± 0.9a | |
| Range (min-max) | 1 - 182 | 0.2 - 14 | 0.9 - 116 | 0.2 - 3 | ||
| Alanine transaminase (ALT), IU/L | ||||||
| Mean | 95.7 ± 70.6 | 29.0 ± 31.4a | 13.7 ± 8.6 | 196.4 ± 272.4 | 19.9 ± 7.9b | 18.4 ± 3.9b |
| Range (min-max) | 15.8 - 481.9 | 3.8 - 287.5 | 3.3 - 45.7 | 13-863 | 6 - 36 | 11 - 26 |
| Aspartate transaminase (AST), IU/L | NA | NA | ||||
| Mean | 73.6 ± 53.3 | 24.4 ± 9.3a | 108.4 ± 90.4 | 19.4 ± 2.5b | ||
| Range (min-max) | 15.4 - 301.3 | 11 - 72 | 19 - 301 | 14 - 23 | ||
| Gamma glutamyl transferase (γ-GT), IU/L | NA | NA | ||||
| Mean | 98.9 ± 119.3 | 32.5 ± 33.2a | 135.3 ± 149.2 | 22.1 ± 16.1b | ||
| Range (min-max) | 15 - 756 | 12 - 251 | 19 - 447 | 12 - 64 | ||
| International normalised ratio (INR) | NA | NA | ||||
| Mean | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.1 | ||
| Range (min-max) | 0.9 - 2.5 | 0.89 - 2.3 | 0.9 - 1.5 | 0.9 - 1.2 | ||
| Creatinine, µmol/L | NA | NA | ||||
| Mean | 69.1 ± 15.8 | 69.8 ± 19.3 | 101.5 ± 57.5 | 81.9 ± 11.6 | ||
| Range (min-max) | 25 - 141 | 44 - 196 | 63 - 271 | 70 - 106 | ||
| Albumin, g/L | NA | NA | ||||
| Mean | 42.6 ± 5.1 | 42.7 ± 4.4 | 35.3 ± 4.9 | 40.5 ± 4.1 | ||
| Range (min-max) | 28 - 53.6 | 30.8 - 51.4 | 27.7 - 42.8 | 31.1 - 44.5 | ||
| Bilirubin, µmol/L | NA | NA | ||||
| Mean | 17.2 ± 9.4 | 19.0 ± 13.1 | 31.8 ± 33.2 | 11.9 ± 5.2 | ||
| Range (min-max) | 3.5 - 61 | 3.7 - 76 | 8 - 127 | 5 - 22.6 | ||
| Platelets (PLT), × 109/L | ||||||
| Mean | 143.2 ± 70.3 | 148.2 ± 70.7 | 128 ± 61 | 225.4 ± 95.4 | 258.5 ± 137.5 | 248.8 ± 111.6 |
| Range (min-max) | 14 - 391 | 48 - 428 | 40 - 338 | 77 - 366 | 127 - 560 | 103 - 407 |
| Haemoglobin (Hb), g/L | ||||||
| Mean | 145.5 ± 16.4 | 131.5 ± 17.2 | 142 ± 17 | 130 ± 20.5 | 129.6 ± 20 | 140 ± 26.8 |
| Range (min - max) | 102 - 180 | 72 - 171 | 92 - 175 | 93 - 163 | 91 - 153 | 82 - 175 |
aP ≤ 0.001.
bP < 0.05.
3.3. Safety
There were no deaths during the treatment and follow-up period; 53 (42.7%) of non-transplant patients reported at least 1 adverse event. Three patients discontinued the treatment prematurely due to AEs (all of them received ribavirin). One patient discontinued the treatment because of psoriasis exacerbation on the day 3, one patient - because of exacerbation of depression at the week 5, and one patient - due to elevation of liver transaminases at the week 9.
The most common AEs were asthenia (25.8%), fatigue (16.1%), skin pruritus (12.9%), dyspepsia (11.3%), and insomnia (9.7%) (Table 5). AEs were mostly mild and did not require medical intervention. AEs occurred more frequently in patients treated with ribavirin.
| Adverse Event | Non-Transplant Patient | Liver Transplant Recipient, N = 10 | ||
|---|---|---|---|---|
| All, N = 124 | RBV(+), N = 95 | RBV(-), N = 29 | ||
| Any adverse events | 53 (42.7%) | 47 (49.5%) | 6 (20.7%)a | 8 (80%) |
| Asthenia | 32 (25.8%) | 29 (30.5%) | 3 (10.3%)a | 4 (40%) |
| Fatigue | 20 (16.1%) | 18 (18.9%) | 2 (6.9%) | 4 (40%) |
| Skin itching | 16 (12.9%) | 16 (16.8%) | 0a | 1 (10%) |
| Dyspepsia | 14 (11.3%) | 13 (13.7%) | 1 (3.4%) | 2 (20%) |
| Insomnia | 12 (9.7%) | 10 (10.5%) | 2 (6.9%) | 0 |
| Joint, lower limbs pain | 9 (7.3%) | 9 (9.5%) | 0 | 1 (10%) |
| Headache | 9 (7.3%) | 8 (8.4%) | 1 (3.4%) | 1 (10%) |
| Mood disorders | 8 (6.5%) | 8 (8.4%) | 0 | 0 |
| Rash | 6 (4.8%) | 5 (5.3%) | 1 (3.4%) | 1 (10%) |
| Dizziness | 5 (4.0%) | 5 (5.3%) | 0 | 0 |
| Cough | 0 | 0 | 0 | 1 (10%) |
| Chest discomfort | 0 | 0 | 0 | 1 (10%) |
| Haemoglobin | ||||
| 80 - 100 g/L | 5 (4%) | 5 (5.3%) | 0 | 5 (50%) |
| < 80 g/L | 1 (0.8%) | 1 (1.1%) | 0 | 3 (30%) |
| Bilirubin | ||||
| ↑3 - 10xULN | 10 (8.6%) | 10 (10.5%) | 0 | 1 (10%) |
| ALT | ||||
| ↑5xULN | 5 (4%) | 5 (5.3%) | 0 | 0 |
aP < 0.05.
RBV, ribavirin; ULN, upper limit normal; ALT, alanine transaminase
The incidence of laboratory abnormalities was uncommon in non-transplant patients and occurred exceptionally in those receiving ribavirin (Table 5, Figure 5). Because of abnormal laboratory test results, ribavirin dose was reduced in 27 (28.4%) non-transplant patients and 3 (30.0%) liver transplant recipients; ribavirin was discontinued in 10 (10.5%) non-transplant patients and 2 (20.0%) liver transplant recipient.
In 6 non-transplant patients and 8 liver transplant recipients, the haemoglobin level dropped below 100 g/L. None of such patients received erythropoietin or blood transfusion.
4. Discussion
Randomized controlled clinical trials are the gold standard to establish the efficacy and safety of any medicinal product. However, clinical trials are conducted under standardized conditions in homogenous patient populations, which is very different from real world situation. Due to differences between patients included in the controlled studies and the ones encountered in everyday life, extrapolation of clinical trial results to the entire patient population is difficult. Real-world studies are conducted in a heterogeneous mixture of patients, often older and with different comorbidities and risk factors. Hence, real-life data better reflect the target population and the real efficacy and safety of many drugs. For these reasons, real-world studies gain more importance in understanding treatment effectiveness and safety profiles in the general patient population.
Since the marketing authorisation of OBV/PTV/r and DSV both by the US food and drug administration (FDA) and the European medicines agency in 2015, a number of real-world studies are conducted (22-27). A meta-analysis of 20 unique patient cohorts across 25 studies encompassing 5158 patients reported the overall SVR12 rates of 96.8% (95% confidence interval (CI): 95.8-97.7) in GT1-infected patients and 98.9% (95% CI 94.2 - 100) in GT4-infected patients. SVR12 rates were consistently high irrespective of cirrhosis status or prior HCV treatment experience (28).
The current study analysed real-world effectiveness of OBV/PTV/r + DSV ± RBV in 134 patients with HCV GT-1 infection treated in 3 health care centres in Lithuania and Latvia. The SVR12 rate was 120 (96.8%) in non-transplant patients and 10 (100.0%) in liver transplant recipients. The study population included a considerable number of treatment-experienced patients (73%) and cirrhotic patients (57%). All the same, more than 90% of them achieved SVR12. Thus, the current study results were consistent with high SVR12 rates (97%-100%) noted earlier in real-world studies (25-27).
The 3 patients that failed to achieve SVR12 were treatment-experienced and 1 was treatment-naïve. Of them, 3 were infected with HCV GT1b and 1 with GT1a/b. Three patients had liver cirrhosis. Patients with cirrhosis and the ones that failed previous antiviral therapies were considered as difficult-to-treat population (12, 18, 19).
The frequency of discontinuation due to adverse events in the current study was low (2.2%) and similar to those of the other real-world studies (~2.5%) (24, 28). Adverse events reported in the current study were mostly mild and more frequent in liver transplant recipients and in patients receiving ribavirin.
As for all real-life studies, the current study had a few inherent limitations and its results should be interpreted with caution. Namely, the study was uncontrolled, retrospective, and there was no external monitoring of the collected data. Clinical examinations were restricted to the ones performed in a routine manner. In addition, the study included a relatively small number of patients and this precluded more thorough subgroup analyses.
4.1. Conclusions
In conclusion, the current real-life study demonstrated the effectiveness and safety of OBV/PTV/r + DSV ± RBV to treat the heterogeneous population of patients with HCV GT1 infection including the ones with cirrhosis, liver transplant recipients, and patients who failed previous antiviral therapies.