1. Background
Autoimmune hepatitis (AIH) is a relatively uncommon immune-mediated liver disease characterized by an increase in circulating autoantibodies, hypergammaglobulinemia, elevated serum transaminase levels, and necrosis of the liver (1). Although the exact pathogenic mechanisms of AIH are still unknown, different studies have revealed that a close interplay between both environmental and genetic factors promote loss of tolerance to liver antigens (2).
Autoreactive T lymphocytes and their inflammatory cytokines are key players in the onset and progression of autoimmune liver diseases and facilitate the liver injury. In patients with AIH, liver damage and autoimmunity of this organ is likely to be mediated by autoreactive T cells (3). Several studies have demonstrated the importance of T cells in AIH. T cells can participate in the disease process through different ways. One important mechanism is the secretion of proinflammatory cytokines like IFN-γ (4, 5).
Since uncontrolled expression of IFN-γ can worsen AIH, it is anticipated that its inhibition may offer a new treatment option for these patients. IFN-γ inhibition can be achieved by different strategies such as neutralization of cytokine, blockage of cytokine receptor, or activation of anti-inflammatory pathways (6, 7).
Recently, small interfering RNAs (siRNAs) show promise in treating multiple autoimmune conditions due to sequence-specific gene silencing. siRNA technology can provide valuable solutions to support patients with severe active disease that has failed to respond to conventional treatments; many studies demonstrated successful application of siRNA (8, 9).
In the current work, the IFN-γ was knocked down with siRNA. Hiperfect-based siRNA delivery was optimized for human peripheral blood mononuclear cells (PBMCs) and showed for the first time anti-cytokine therapy with siRNA technology in AIH. The results of the present study may be helpful for future clinical development with siRNA-base specific treatment.
2. Methods
2.1. Ethics
This study was complied with the declaration of Helsinki principles and approved by ethics committee of Tehran University of Medical Sciences and ethical committee at the national institute of genetic engineering and biotechnology in Iran (ethics code: IR.NIGEB.EC.1395.5.30.C). Blood samples from patients were collected after each subject signed the written informed consent. These subjects were diagnosed as AIH patients in digestive disease research center (DDRC) of Shariati hospital (10). All of the subjects were medication free. AIH patients were diagnosed if they had chronically elevated aminotransferase, hypergammaglobulinemia, positive auto-antibodies, histopathological features compatible with autoimmune hepatitis on liver biopsy, and absence of viral, metabolic, and drug-induced liver disease (Table 1).
| Naive | Age (y) | Sex | AST (IU/L) | ALT (IU/L) | IgG | ANA | SMA | Anti-LKM-1 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | + | 28 | F | 378 | 125 | 2416 | + | + | - |
| 2 | + | 36 | F | 602 | 593 | 4514 | + | + | - |
| 3 | + | 35 | F | 520 | 550 | 3119 | + | - | - |
| 4 | + | 56 | F | 118 | 102 | 2656 | + | - | - |
| 5 | + | 29 | M | 360 | 800 | 2430 | + | - | - |
| 6 | + | 36 | F | 132 | 186 | 2007 | + | - | - |
2.2. Cell Culture
2.2.1. Isolation of PBMCs and Stimulation of Human T lymphocytes Proliferation
Approximately 10 mL of blood was obtained from each subject. PBMCs were isolated by Ficoll (GE Healthcare, Uppsala, Sweden) density gradient centrifugation of heparinized whole blood according to standard protocols. Isolated PBMCs were individually cultured in RPMI1640 medium (Sigma-Aldrich, USA) supplemented with 20% heat inactivated fetal bovine serum (FBS) (Gibco, USA), 100 U/mL penicillin and 100 µg/mL streptomycin (Sigma-Aldrich, USA) for 24 – 48 hours at 37°C in a humidified atmosphere with 5.0% CO2 and 95% air. PBMCs were stimulated with 1% phytohemaglotinin (PHA) (Gibco, USA) for mainly amplification of T cells. After this time, PHA-activated PBMCs were collected by a 10 minutes centrifugation at 395 g at 4°C and removed adherent cells. Obtained cells were resuspended in warm complete RPMI1640 medium (with 10% FBS) and counted using trypan blue and hemocytometer. Counted cells were seeded in 24-well plate for transfection. For suspension, cells should be seeded 2 × 105 cells/ well.
2.2.2. siRNA Synthesis and Transfection
The sense-strand sequence of siRNA designed to target the IFN-γ was 5’-GAAACGAGAUGACUUCGAATT-3’. siRNA, with 21-nt length and 5’-Fluorescein (6 FAM) modification on sense strand, was synthesized by Qiagene company. In this study, AllStars negative control siRNA was used (Qiagene, Germany). siRNA transfection was carried out as suggested protocols of Qiagene company.
First, dose response and time study were performed. 50, 100, 150, and 200 nM concentrations of siRNA were studied for transfection. Above mentioned concentrations were evaluated for 12, 24, 48, and 72 hours time period.
siRNA transfection was done by the fast-forward protocol. First, 2 × 105 cells were seeded per well of a 24-well plate in 100 µL RPMI1640 medium with 10% FBS and 1% Penicillin-Streptomycin. For the complex formation, siRNA was diluted in warm RPMI1640 medium without serum. This is due to the fact that serum inhibits the complexes’ formation and causes a poor gene knockdown efficiency. Then, 6 µL HiPerFect transfection reagent was added to the diluted siRNA and mixed. For the formation of transfection complexes, samples were incubated for 10 - 15 minutes at room temperature. Then, the complexes were added drop-wise onto the seeded cells. The cells were incubated for 6 h with the transfection complexes at normal growth conditions. Subsequently, 400 µL RPMI1640 complete medium was added to the cells per well and incubated until analysis of gene silencing at the mRNA and protein levels. Analysis of transfection efficiency in cells was done by fluorescence microscopy. This is due to the fact that siRNA harbors 5’-Fluorescein (6 FAM) modification; transfected cells represent green fluorescence.
2.2.3. Assessment of Cell Death in Treated Cultures by Acridine Orange/Ethidium Bromide (AO/EB) Double Staining Assay and Flow Cytometry
The study of cell death was done by AO/EB double staining as previously described (11). Briefly, transfected and untransfected cells were stained with dual fluorescent staining solution (1 µL) containing 100 µg/mL acridine orange and 100 µg/mL ethidium bromide (Sigma-Aldrich, USA). Then, the stained cells were examined under fluorescence microscope. Viable cells were determined with bright green nuclei, whereas apoptotic and necrotic cells had orange to red nuclei.
In order to quantify the cell death assessment, the flow cytometry was performed. After IFN-γ-siRNA transfection, the cells were examined to assess the incidence of apoptosis and necrosis of all transfected cells. For this purpose, the Annexin V-FITC/PI Kit (BioLegend, California) was used. After transfection, the cells were collected, washed with PBS, resuspended in annexin V binding buffer 1X, incubated with annexin V-FITC/PI in the dark for 15 minutes, and finally analyzed with flow cytometry (BD FACSCalibur flow cytometer, USA). The data were analyzed using FlowJo software.
2.3. Real-Time PCR
Total RNA was isolated from transfected/control cells using a Hybrid R (GeneALL, Seoul, Korea), according to the manufacturer’s instructions. First-strand cDNA synthesis was performed using oligo- (dT) primer and RevertAid First Strand cDNA synthesis kit (Thermo scientific, US). GAPDH was used as control gene. GAPDH and cytokine gene transcripts (IFN-γ) were quantified by real-time PCR using a SYBR Premix EX Taq II (Takara, Japan) on Rotor Gene 6000 thermal cycler (Corbett Life Science, Australia). PCR was performed in triplicate with total volume of 10 µL containing 1 µL of cDNA and 5 µL of SYBR Premix with 0.5 µL of forward and reverse primers. Amplification conditions were as follow: step 1. One cycle of initial denaturation (95°C for 5 minutes), and step 2. 45 cycles of amplification (95°C for 5 seconds and 60°C for 45 seconds (IFN-γ) and 62°C for 20 seconds (GAPDH)). Primers were used for IFN-γ (F: 5’-TCGGTAACTGACTTGAATG-3’; R: 5’-ATTACTGGGATGCTCTTC-3’) and GAPDH (F: 5’-GCAGGGGGGAGCCAAAAGGGT-3’; R: 5’-TGGGTGGCAGTGATGGCATGG-3’). Relative mRNA expression was calculated using Relative Expression Software Tool (REST2009, Qiagene).
2.3.1. Analysis of Gene Knockdown in Protein Level by Intracellular Flow Cytometry
After siRNA transfection, detection of intracellular IFN-γ was performed by intracellular flow cytometry staining. Isolated PBMCs from AIH patients were cultured in RPMI1640 complete medium and siRNA transfection; 10 µgr/mL brefeldin A (BioLegend, California) was added to cells in the last 4 - 6 hours of cell culture. Brefeldin A inhibits IFN-γ secretion. After the incubation period, transfected cells were collected, washed twice with PBS, and stained with specific antibodies. For cell surface staining, the cells were incubated with 5 µL CD3 FITC (BioLegend, California), in 4°C. The cells were washed and fixed in 0.5 mL/tube fixation buffer (BioLegend, California) in the dark for 20 minutes at room temperature. Then, the cells were centrifuged at 350 g for 5 minutes. For permeabilization, fixed cells were resuspended in permeabilization wash buffer (BioLegend, California) and centrifuged at 350 g for 5 minutes. Finally, for intracellular staining, fixed/permeabilized cells were resuspended in residual permeabilization wash buffer and fluorophore-conjugated antibody of IFN-γ (BioLegend, California) was added and incubated for 30 minutes at 4°C in the dark. Finally, data obtained from stained and fixed lymphocyte samples were analyzed using FlowJo.software.
2.4. Statistical Analysis
All results are presented as mean ± SEM from triplicate performed experiments. Changes in the expression ratio after siRNA transfection were evaluated for significance using Student’s t-test. Statistical significance was determined as P < 0.05.
3. Results
3.1. Selection of the Optimal Dose of IFN-γ-siRNA After In Vitro Transfection of Stimulated PBMCs from AIH Patients
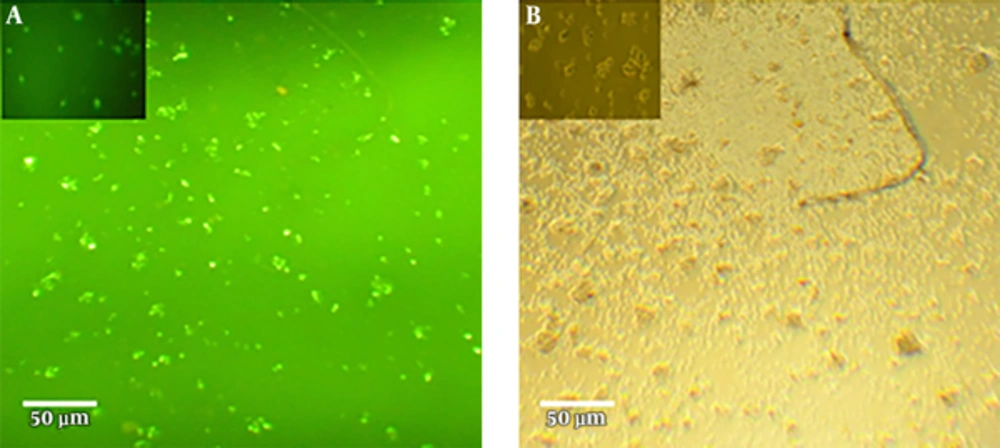
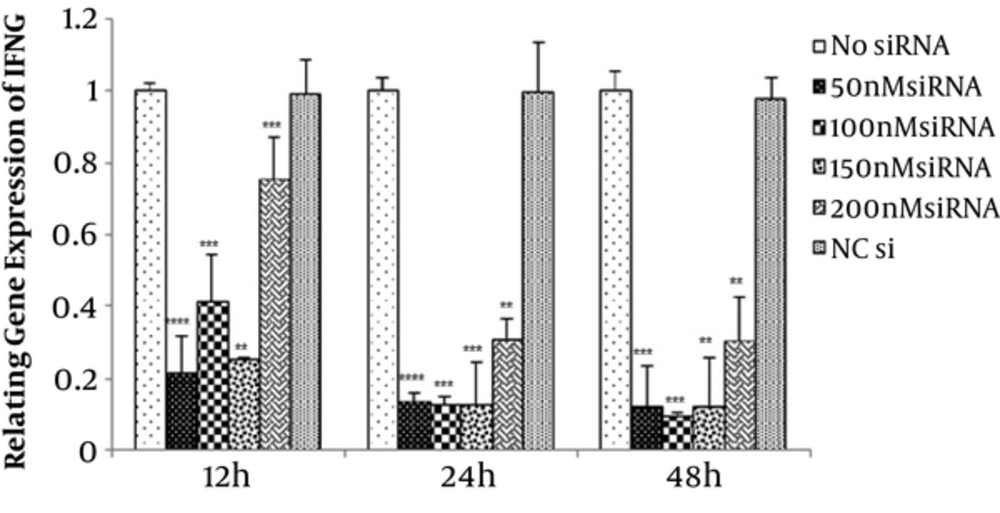
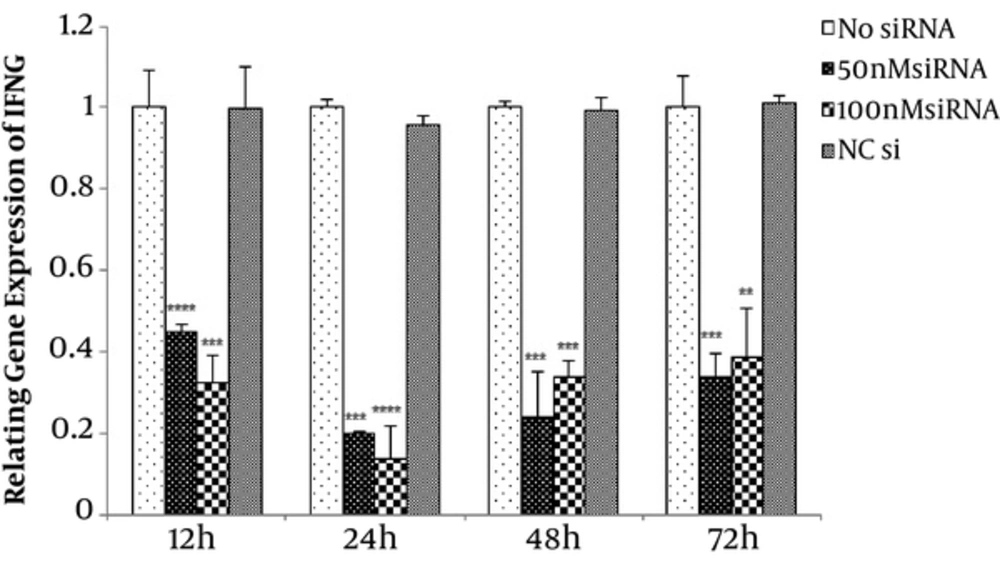
To find the optimal dose, 50, 100, 150, and 200 nM concentrations of IFN-γ-siRNA were considered for 12, 24, 48, and 72 hours time periods. Efficiency of transfections were evaluated by fluorescence microscopy, which is shown in Figure 1. Studied samples revealed 60% - 70% transfection rate. In order to verify the gene silencing and selection of the most effective dose of IFN-γ-siRNA, quantitative gene expression was done. Results showed down-regulation of IFN-γ in all of the siRNA doses (P < 0.05) (Figure 2). For largely avoiding of off-target effects, lowest IFN-γ-siRNA concentrations were selected, which were 50 and 100 nM for the aforesaid cells in this study.
Efficient siRNA transfection in PHA-activated PBMCs. (a) PHA-activated PBMCs were treated with 50 nM IFN-γ-siRNA (harbored 5’-Fluorescein (6 FAM) modification (green)) for 24 hours. (b) untransfected cells that have not revealed any green fluorescence. siRNA transfection was carried out in normal RPMI1640 medium with 10% FBS.
Assessment of optimal dose of IFN-γ-siRNA for transfection. 50, 100, 150 and 200 nM concentrations of IFN-γ-siRNA and negative control were considered for 12, 24 and 48 hours time period. Results are showing down-regulation of IFN-γ in all of the siRNA doses (**P < 0.01, ***P < 0.001, ****P < 0.0001). For avoiding of off target effects, 50 and 100 nM concentrations of IFN-γ-siRNA were selected.
After selecting most effective and low risk doses of IFN-γ-siRNA, this study proceeded in other AIH patients. Stimulated PBMCs from AIH patients were transfected with 50 nM and 100 nM concentrations of IFN-γ-siRNA.
3.2. IFN-γ-siRNA Treatment Did not Impose Negative Effects on Cell Viability
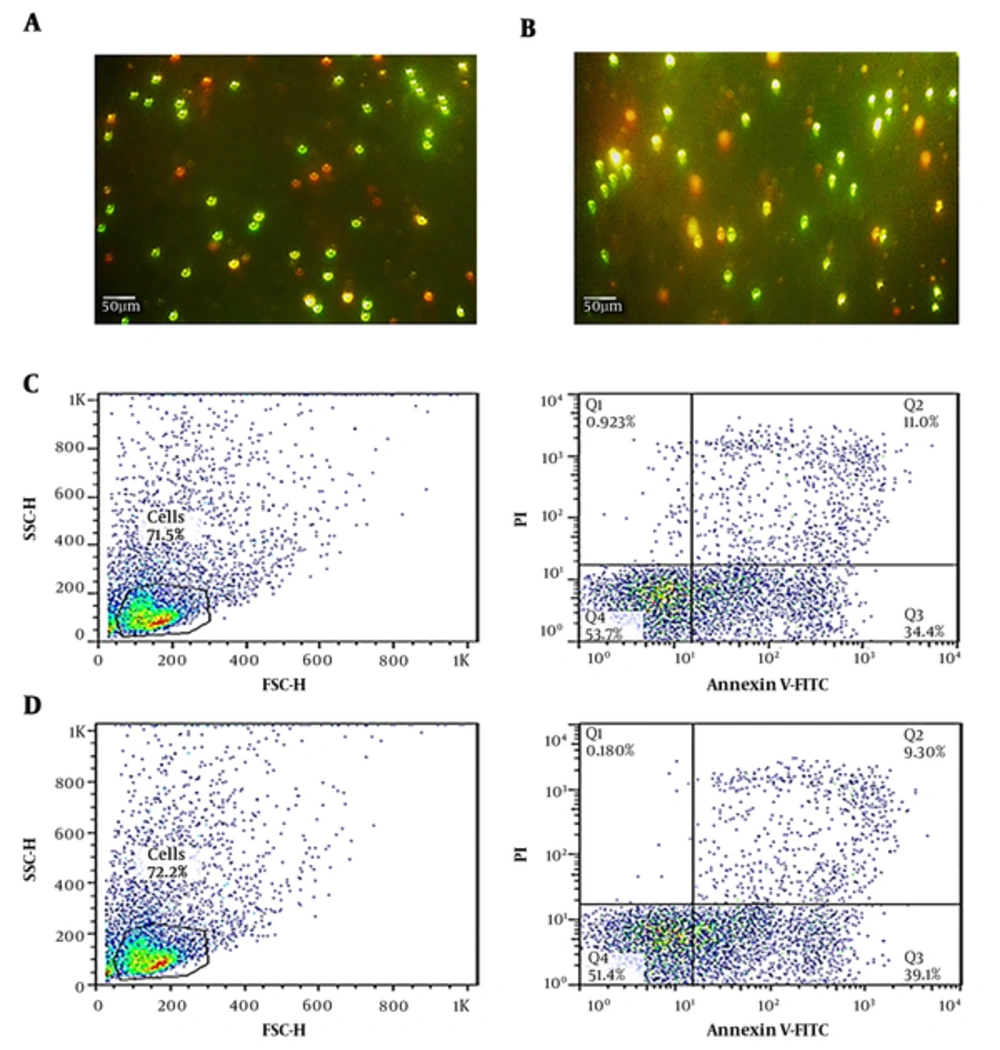
Cell viability of the transfected cells evaluated by AO/EB double staining and flow cytometry. Results of staining showed no significant differences between transfected and untransfected cells (Figure 3).
Comparative analysis of cell death between isolated PBMCs from one AIH patient that had been transfected with IFN-γ-siRNA and untransfected controls. (a and b) The cell death assessment using AO/EB double staining. (c and d) The cell death assessment using flow cytometry. (a, c) untransfected cells. (b, d) IFN-γ-siRNA transfected cells. The number of apoptotic and necrotic cells did not reveal significant difference between transfected and untransfected cells.
In flow cytometric analysis, after 24 hours, the incidence of cell death was assessed. The percentage of apoptosis was defined as the number of cells positive for annexin V-FITC, however, negative for propidium iodide; the percentage of necrosis as the number of cells was positive for both of them. Results are shown in Figure 3, presenting no statistical significance after transfection.
3.3. Suppression of IFN-γ at the both of mRNA and Protein Levels after siRNA Transfection
Monitoring gene silencing at the mRNA level was done with real-time PCR. The results were summarized in Figure 4. As expected, IFN-γ-siRNA treatment significantly downregulated IFN-γ mRNA compared with untransfected control cells (P < 0.05). Downregulation of IFN-γ mRNA was shown in all of the doses and times (Figure 4). Expression of IFN-γ was normalized to expression of GAPDH as housekeeping gene. AllStars negative siRNA was used as control. Gene expression analysis of this negative control siRNA transfection revealed no significant statistical differences compared with untransfected control cells.
Quantitation of IFN-γ mRNA after transfection in the AIH patients by real-time PCR. Stimulated PBMCs were transfected with 50 and 100 nM of IFN-γ-siRNA for 12, 24, 48 and 72 hours time period. IFN-γ-siRNA treatment significantly reduced IFN-γ mRNA. The analysis was performed for six different donors (**P < 0.01, ***P < 0.001, ****P < 0.0001).
Gene silencing analysis at the protein level is the most comprehensive way of showing that a gene has been downregulated. The protein amount of IFN-γ was measured with intracellular staining and flow cytometry.
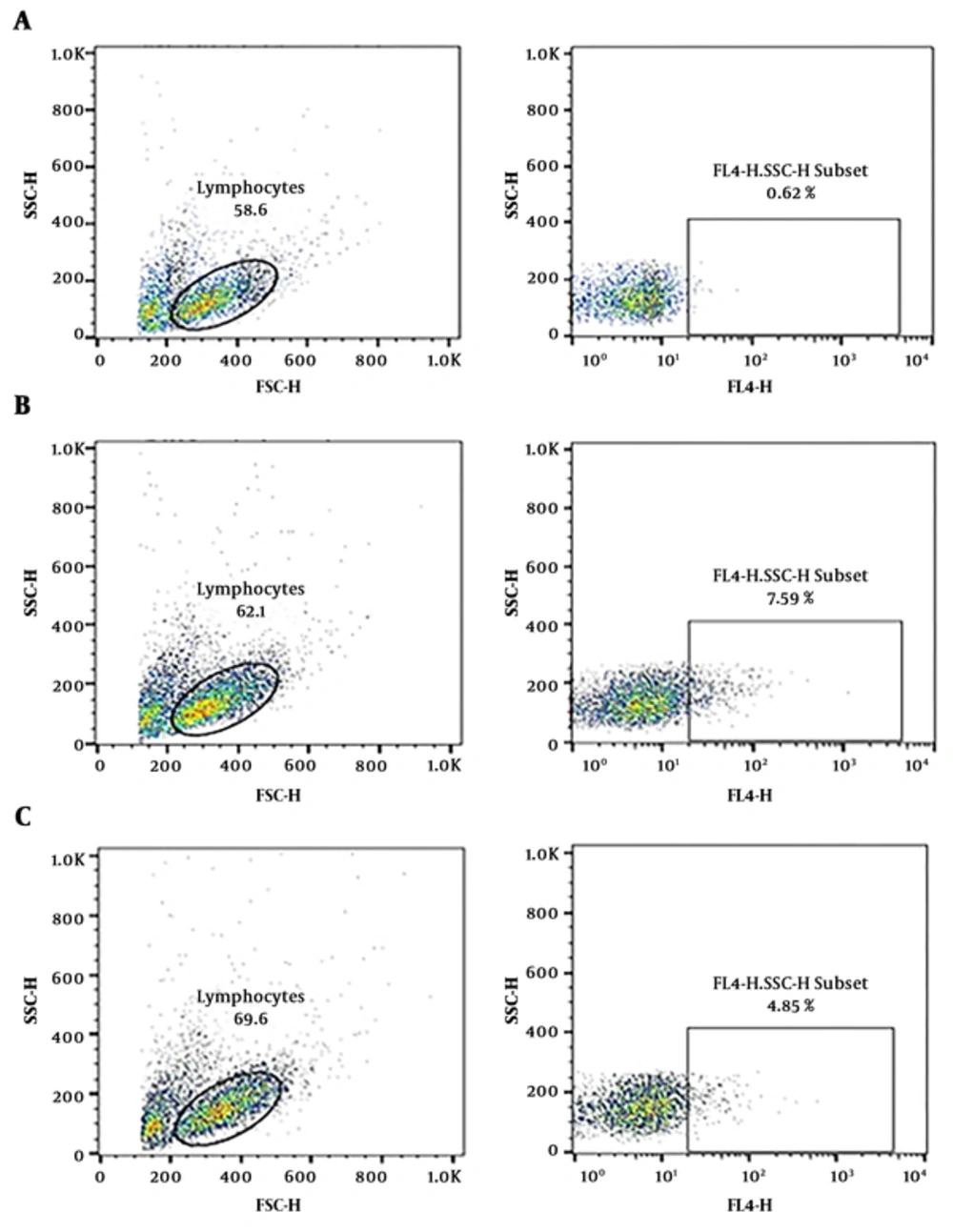
Flow cytometric analysis of the IFN-γ-siRNA transfected cells confirmed the results of the real-time quantitative PCR. These results demonstrated a significant decrease in protein production of transfected cells. Protein amount of IFN-γ in untransfected cells was 7.59% vs. 4.85% in IFN-γ-siRNA transfected cells. A total of 50% reduction in protein amount of IFN-γ is clearly shown in Figure 5. The results of mRNA and protein assessments proved that IFN-γ-siRNA correctly targeted the mRNA.
Decrease of the IFN-γ secretion in isolated PBMCs from one AIH patient after siRNA transfection. (a) unstained cells. (b) untransfected cells. (c) transfected cells. PBMCs were isolated from one AIH patient and after IFN-γ-siRNA transfection stained with CD3FITC and fluorophore- conjugated antibody for IFN-γ respectively. The assessment of IFN-γ secretion in gated lymphocytes was revealed nearby 50% decrease in IFN-γ-siRNA transfected cells.
4. Discussion
In the current study, a new approach for the treatment of AIH using IFN-γ-siRNA with non-viral delivery system was proposed. The results revealed that IFN-γ-siRNA was delivered into PHA-activated PBMCs efficiently and resulted in a significant reduction of IFN-γ as an important proinflammatory cytokine in pathogenesis of AIH patients. This method is one approach to immunotherapy and demonstrate the potential therapeutic applications of RNA interference (RNAi) for AIH. IFN-γ inhibition may also be potentially useful in patients who suffer from side effects caused by immunosuppressive drugs (12).
As mentioned above, the remission of more than 80% AIH patients achieves by receiving prednisolone and azathioprine and corticosteroid therapy. These immunosuppressive drugs have many adverse effects in long-term use that is serious for individuals (13).
Like other autoimmune disorders, AIH has also an unknown etiology and in turn, a relatively blind treatment. Therefore, understanding of the pathogenic mechanisms of AIH at the molecular level is essential in developing new useful therapeutic strategies (14). Achievement to a complete remission that prevents disease progression with lowest possible dose and without side effects is the final goal of AIH treatment (3).
RNAi and siRNA-mediated gene knockdown are post-transcriptional gene silencing strategies. These powerful tools have revolutionized basic biomedical science and applied for disease treatment in a sequence-specific manner (12). Up to now, 21 different siRNA drugs have been evaluated in clinical trial setting and used for genetic disorders, such as, Alzheimer’s, Parkinson’s, and cancer (15, 16).
Our pervious study revealed that AIH is a T cells-dependent autoimmune disease and newly-diagnosed patients showed high levels of IFN-γ expression (5). The current study tried to expand our pervious results and propose a therapeutic way for AIH. Anti-cytokine therapy with a potent siRNA was approached for targeting of IFN-γ, which shows abnormal rise in patients. Increased IFN-γ expression can result in development of autoimmunity and end organ damage. This damage is due to infiltration of IFN-γ secreting T-cells resulting in macrophage activation, inflammation and tissue damage (17, 18).
Several studies have tried to determine the effectiveness of anti-IFN-γ therapy. Application of monoclonal antibodies against IFN-γ was reported in patients with Crohn’s disease (19) and systematic lupus erythematosus, which resulted in some efficacy in patients (17).
In the current study, IFN-γ-siRNA were used to selectively inhibit this cytokine and hypothesized that siRNA-mediated gene silencing could knockdown IFN-γ mRNA and in turn protein production and inhibit the progression of AIH. The level of IFN-γ mRNA showed a significant decrease (relatively 50% - 80%) after IFN-γ-siRNA transfection of PBMCs (P < 0.05). Intracellular staining and flow cytometry results validated this effect too. The protein amount of IFN-γ was also decreased in the treated cells.
The current results were comparable, if not superior, to those of other publications that presented a therapeutic strategy and suppressing autoreactive T cells for AIH or other autoimmune diseases (12, 20, 21). We proposed siRNA based therapeutic tool. The ease of synthesis, low production cost (compared with protein or antibodies therapies), and having favourable pharmacokinetic properties for delivery to a wide range of organs are the advantages of this method (22, 23). Similar strategies can be used to knock down other critical molecules associated with autoimmunity in liver and other organs.
In summary, our findings demonstrated that IFN-γ-siRNA could be useful for anti-cytokine therapy in AIH patients. The IFN-γ-siRNA complexes can effectively knock down IFN-γ gene expression and reduced cytokine secretion in vitro. In order to confirm these findings, further studies like in vivo experiments are required. Finally, siRNA, as the easy-to-use technology, may present a promising clinical application of RNAi as powerful strategy for effective molecular therapy of many autoimmune diseases. Moreover, the identification of irregular immune molecules raises the possibility of using such methods for individualized therapy of autoimmune diseases.