1. Background
Hepatitis B virus (HBV) is the prototype of the Hepadnaviridae (hepatotropic DNA virus) family and has a strong preference for infecting liver cells (1). HBV infection is a major global health problem with approximately 350 million people chronically infected worldwide, especially in Asia, accounting for a large morbidity and mortality of liver disease (2-4). HBV infection leads to the liver injury and inflammation and gradually progresses to liver fibrosis, cirrhosis, and even hepatocellular carcinoma (HCC) (5, 6). The spectrum of the disease and the history of chronic HBV infection are various and variable, ranging from inactive carrier state to progressive chronic hepatitis B (CHB) (7). The primary chronic HBV infection in adults and children can be either symptomatic or asymptomatic, and the long-term HBV infection increases the risk of developing fibrosis and HCC (8). Therefore, it is very important and necessary to diagnose the liver injury precisely during the chronic HBV infection in the early stage and provide a reasonable treatment scheme to prevent the progression of liver disease.
Up to now, the liver biopsy is still considered as the gold standard for the staging of hepatic inflammation and fibrosis. The application of liver biopsy is limited because of its high cost, invasiveness, and the association with the risk of complications. Furthermore, the diagnostic accuracy of liver biopsy decreases due to sampling errors and a significant rate of intra- and inter-observer variability (9-11). Currently, some novel non-invasive methods are widely used to assess the severity of liver injury, including serum markers, such as alanine transaminase (ALT) (12), aspartate aminotransferase (AST) (13), Cytokeratin-18 (CK-18) (14), and aspartate aminotransferase to platelet ratio index (APRI) (13), and imaging techniques such as transient elastography (TE) (15) and magnetic resonance elastography (MRE) (16).
Cytokeratin-18 (CK-18) is a main cytoskeletal protein in hepatocytes and other epithelial cells. CK-18 could be released into serum by the dying cells as a cell death signal (17). Two forms of CK-18 are observed in the serum including the whole CK-18 protein (CK-18 M65) and the caspase 3-cleaved fragment (CK-18 M30). CK-18 M65 is used to evaluate the total cell death whereas CK-18 M30 is specifically used to evaluate the apoptotic-associated cell death (18). Accumulated evidence suggests that hepatocyte apoptosis plays a vital role in chronic liver disorders (17, 19). Yang et al. found that the serum CK-18 levels might be a risk factor for non-alcoholic steatohepatitis (NASH), chronic hepatitis C (CHC), and chronic hepatitis B (CHB) (all P < 0.05) through conducting a meta-analysis of eight case-control studies (20). Some reports also showed that the plasma levels of CK-18 M30 were associated with the progression of non-alcoholic steatohepatitis (NASH) in non-alcoholic fatty liver disease (NAFLD) patients (21, 22). Recently, the associations between serum levels of CK-18 M30 and the progression of the chronic HBV infections were investigated. However, the results have been highly variable. Therefore, we performed a meta-analysis to explore the association between serum CK-18 M30 levels and chronic HBV infection and the progression of chronic hepatitis B.
2. Objectives
The aim of this study was to investigate the association between serum CK-18 M30 levels and the severity of liver injury in chronic hepatitis B (CHB) patients.
3. Methods
3.1. Search Strategy
A systematic literature search was performed in PubMed, Embase, and Cochrane Library for relevant studies about humans published in English up to August 2017. The following keywords were used during the search: “HBV infection” or “chronic virus hepatitis” or “chronic hepatitis B” or “CHB” and “Cytokeratin-18” or “Keratin-18” or “CK-18” or “CK18.” Studies that were not published as full reports, such as conference abstracts, letters, and case reports were excluded. In addition, a manual literature search was conducted using the references of original manuscripts and reviews to identify additional related studies. We also contacted the authors of studies containing relevant information, who did not report the results necessary for this analysis. The eligible reports were identified by two investigators (CF Li and SS Liu) independently, and controversial studies were resolved through iteration and consensus.
3.2. Study Selection
The study eligibility was also independently determined by the same two investigators (CF Li and SS Liu), and any disagreement was resolved by consensus. Studies meeting the following inclusion criteria were included: (I) studies evaluating the association between serum CK-18 M30 levels and chronic hepatitis B; (II) studies on chronic hepatitis B patients who possessed features including positivity for HBsAg, > 105 copies per mL of serum HBV DNA, and/or elevated ALT levels (more than the upper limit of normal on > 2 separate monthly decision within the last 6 months); (III) articles published with full text and; (IV) studies with sufficient information on the serum CK-18 M30 levels in the cases and controls. The criteria for exclusion were as follows: (I) studies not satisfying the inclusion criteria; (II) letters, case report, conference abstracts, reviews, or meta-analyses; (III) repeated publications or studies with over-lapping data; (IV) studies without control subjects or studies that involved less than 30 participants; (V) studies that included patients co-infected with NAFLD or HCV or acute hepatitis, having alcohol-related liver diseases, or having received antiviral treatments previously.
3.3. Data Extraction and Quality Assessment
Two investigators (CF Li and SS Liu) independently extracted data from eligible studies. Disagreements were resolved by discussion and consensus. For each study, the following information was extracted from each eligible study: the name of the first author, the year of publication, the place of study conduction, the patient age, gender, publication language, the number of cases and controls, serum CK-18 M30 levels in the case and control group, and the detection method of CK-18 M30 in each study. The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of studies enrolled in our meta-analysis (23). The NOS is composed of eight questions with nine possible points that included three aspects: (I) subjects selection: 0 - 4; (II) comparability in subjects: 0 - 2 and; (III) ascertainment for the exposure: 0 - 3. In this meta-analysis, studies receiving 6 or higher scores were regarded as high-quality studies. The heterogeneity test was conducted among these studies using Q-test (P < 0.05 was regarded as statistically significant) and I2 index. A value of 25% - 50% for I2 indicates a low degree of heterogeneity, a value of 50% - 75% indicates a moderate heterogeneity, and a value of > 75% indicates a high heterogeneity. When the I2 value was > 50%, a random-effects model was used to pool the data; otherwise, a fixed-effects model was selected (20). The association between serum CK-18 M30 levels and CHB was estimated by the standard mean difference (SMD) with 95% confidence interval (CI). Sensitivity analysis was performed to investigate the source of heterogeneity by removing a study each time to assess the stability of our results. P < 0.05 was considered statistically difference. The Funnel plot was used to evaluate the possible publication bias using the Begg’s test; P < 0.05 represented the significant asymmetry of the funnel plot. All analyses were performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) and Stata 12.0 (Stata Corporation, Texas, US).
4. Results
4.1. Literature Search
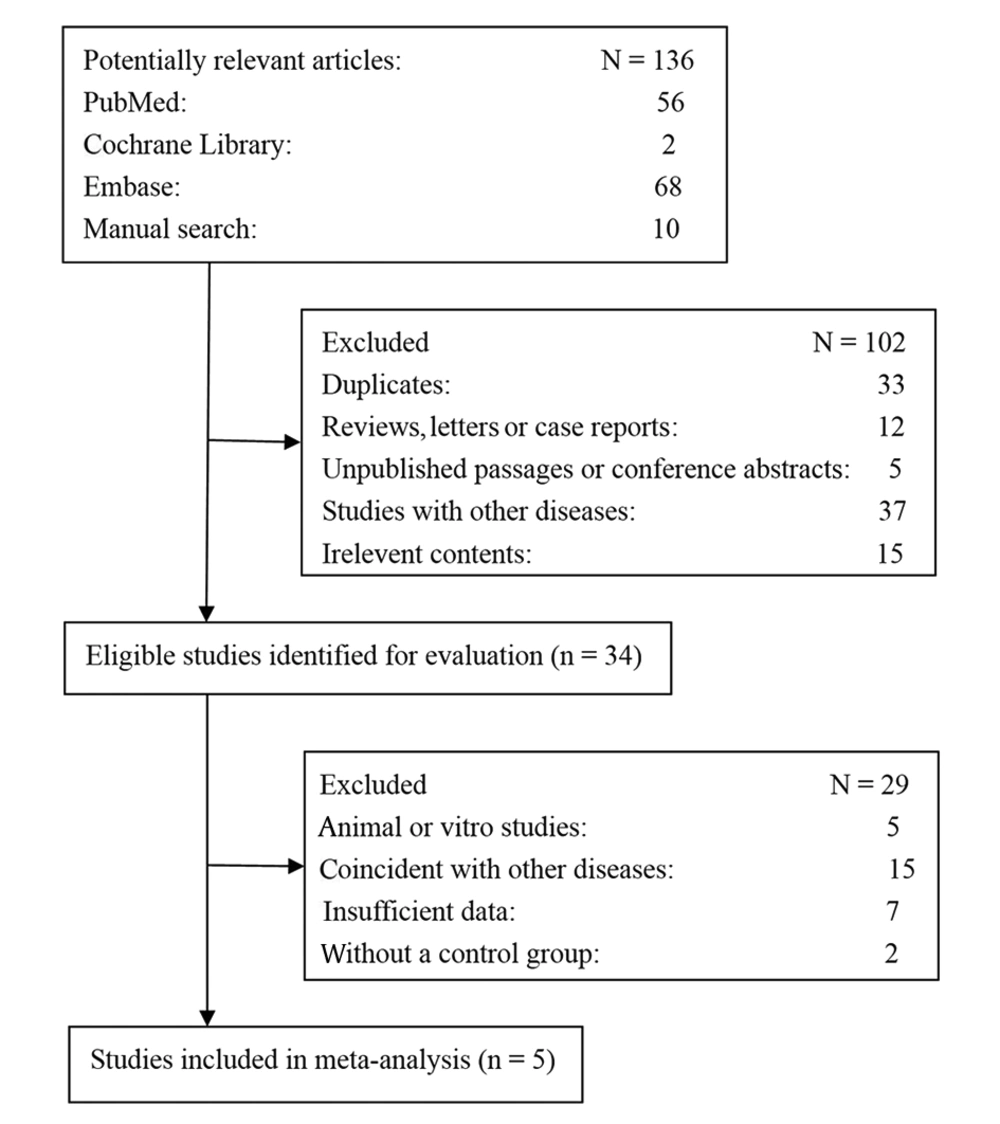
A total of 136 articles were retrieved initially through electronic database searching and manual search. 34 papers were kept after removal of duplicates (n = 33), reviews, letters or case reports (n = 12), unpublished passages or conference abstracts (n = 5), studies with other diseases (n = 37), and studies unrelated to the research topics (n = 15). Furthermore, 29 additional studies were excluded because they were animal or in-vitro studies (n = 5), coincident with other diseases (n = 15), insufficient data (n = 7), or without control groups (n = 2). Eventually, 5 reports were included in the meta-analysis. Figure 1 shows the flowchart of the literature search conducted in this study.
4.2. Characteristics of Included Studies
Five studies published from 2008 to 2017 were eligible for meta-analysis (17, 24-27). The basic features of these studies are shown in Table 1. Four studies were performed in populations of Turkey and one study was performed in populations of Greece. A total of 488 CHB patients, 276 inactive carriers, and 193 healthy controls was included in this study. All the studies were case-control trials; patients in two studies were all negative for HBeAg (24, 25), and one study divided the CHB patients into HBeAg positive and HBeAg negative (17). All the CHB patients were diagnosed by liver biopsy, and the immunohistochemistry (IHC) or ELISA methods were used to test the serum CK-18 M30 levels in the five studies. The results of methodological quality assessment using NOS and the scores of the included articles were between 6 and 7, representing the high quality of studies.
| First Author | Year | Country | Total | Sample Size | Gender, M/F | Age, y | Diagnose Criteria | Method (CK 18 M30) | NOS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHB | Inactive Carriers | Controls | CHB | Inactive Carriers | Controls | CHB | Inactive Carriers | Controls | |||||||
| Papatheodoridis | 2008 | Greece | 145 | 62 (-) | 53 | 30 | 45/17 | 30/23 | NA | 48 ± 15 | 41 ± 13 | NA | Liver biopsy | IHC | 7 |
| Eren | 2010 | Turkey | 172 | 42 (+), 47 (-) | 54 | 29 | 21/26, 22/20 | 28/26 | 17/12 | 45.9 ± 9.7, 47.2 ± 8.5 | 44.7 ± 9.9 | 46.6 ± 9.3 | Liver biopsy | ELISA | 6 |
| Sumer | 2013 | Turkey | 240 | 189 (-) | / | 51 | 132/57 | / | 25/26 | 37.8 ± 12.6 | / | 33.8 ± 9.3 | Liver biopsy | ELISA | 7 |
| Yilmaz | 2016 | Turkey | 88 | 48 | / | 40 | 25/23 | / | 14/26 | 45.66 ± 11.45 | / | 41.67 ± 13.68 | Liver biopsy | ELISA | 7 |
| Balkan | 2017 | Turkey | 312 | 100 | 169 | 43 | 63/37 | 94/75 | 31/12 | 33.54 ± 11.74 | 36.89 ± 10.97 | 31.28 ±7.49 | Liver biopsy | ELISA | 7 |
Characteristics of Included Studies Focused on Serum Levels of CK-18 M30
4.3. Association of the Serum CK-18 M30 Levels with Chronic HBV Infection
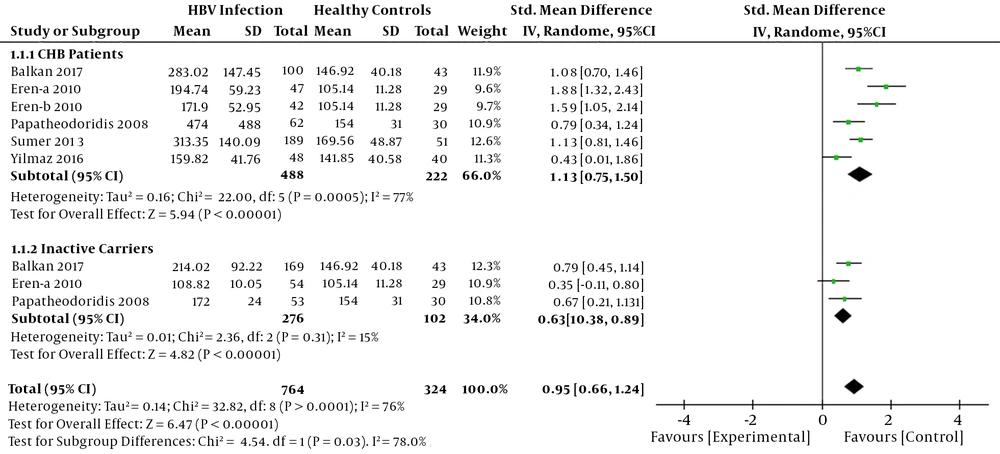
Heterogeneity analysis results (I2 > 50%) showed that there was a significant heterogeneity among the studies; thus, the random-effects model was used. As shown in Figure 2, the elevated serum CK-18 M30 levels were observed in chronic HBV infected patients compared to healthy controls (SMD = 0.95, 95%CI: 0.66 - 1.24, P < 0.001) in this meta-analysis. Based on the phase and the severity of chronic HBV infections, we divided the patients into two subgroups: CHB patients with severe liver injury group and inactive carriers group. The subgroup analysis revealed that serum CK-18 M30 levels were significantly higher in CHB patients with severe liver injury and inactive carriers compared to healthy controls (SMD = 1.13, 95% CI: 0.75 - 1.50, P < 0.001; SMD = 0.63, 95% CI: 0.38 - 0.89, P < 0.001, respectively) (Figure 2).
4.4. Serum CK-18 M30 Levels between CHB Patients and Inactive Carriers
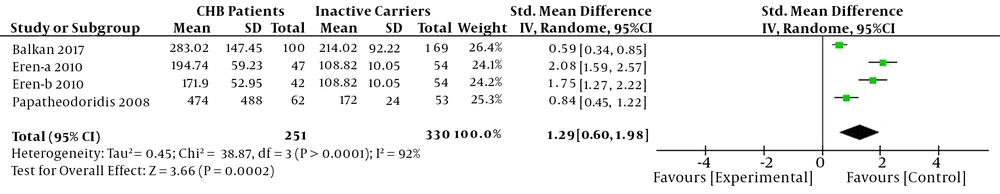
The differences of serum CK-18 M30 levels between CHB patients with severe liver injury and inactive carriers were investigated. A random-effects model was used because a heterogeneity was existed (I2 > 50%). The results showed that CHB patients with severe liver injury had higher serum CK-18 M30 levels than inactive carriers in this meta-analysis (SMD = 1.29, 95%CI: 0.60 - 1.98, P < 0.001) (Figure 3).
4.5. Sensitivity Analysis and Publication Bias
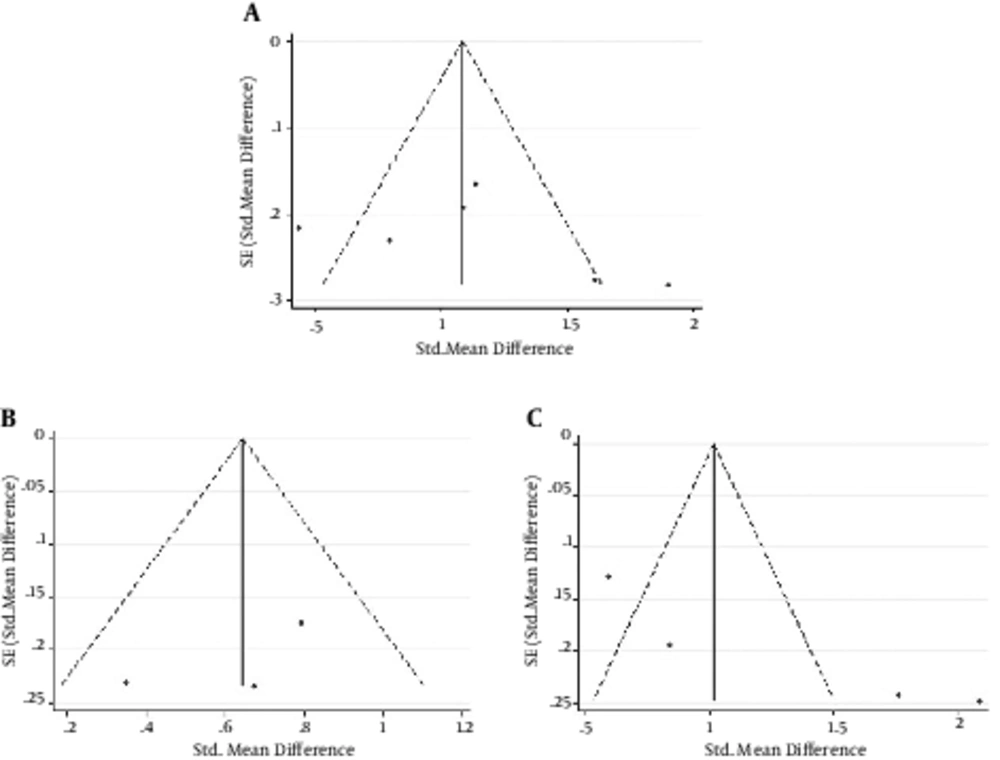
Sensitivity analysis was performed to evaluate the robustness of the meta-analysis. The removal of any single study did not change the overall statistical difference, suggesting that the current meta-analysis data of each group were relatively stable and reliable. Begg’s tests were performed for the publication bias analysis of the groups between chronic HBV infected patients and healthy controls, inactive carriers and healthy controls, and CHB patients and inactive parries. The results showed that no significant publication bias was existed among the studies (P = 0.452; P = 1.000; P = 0.089, respectively) (Figure 4).
5. Discussion
Cytokeratin-18 is as a cytoskeletal protein found mainly in epithelial cells and other epithelial cells plays a key role in maintaining the shape of cells and resisting mechanical stresses (28). Previous research reported that CK-18 is expressed by most types of carcinoma cells, such as breast, prostate, lung, liver, colon, and ovary cells, and could be regarded as a useful serum marker for evaluating the clinical progression of patients with epithelial malignant tumors (29, 30). Recently, the increased serum levels of CK-18 in severe liver diseases were observed suggesting that it may be as a potential serum marker for the diagnosis of clinical liver disease. CK-18 is composed of two molecular forms: the whole CK-18 protein (CK-18 M65) and the caspase-cleaved fragment (CK-18 M30), both of which can be detected in the serum. Accumulated evidence showed that the levels of CK-18 M30 are significantly associated with NASH progression in NAFLD patients of both adults and children (14, 31, 32). Chen et al. reported that the sensitivity and specificity of CK-18 M30 for NASH were 83% and 71% via a meta-analysis of nine studies (33).
The association of serum CK-18 M30 levels with the chronic hepatitis B has well been studied (17, 24-27). This comprehensive meta-analysis investigated the relationship of serum CK18 M30 levels with chronic HBV infection. As shown in the results, the serum CK-18 M30 levels were significantly higher in CHB patients with severe liver injury than in healthy controls (SMD = 1.13, 95% CI: 0.75 - 1.50, P < 0.001). Swiderska et al. reported that the serum CK-18 M30 levels were sensitive and specific for the discrimination of mild, moderate, or severe fibrosis and inflammation even in patients with normal ALT activity (34). In order to investigate the relationship of serum CK-18 M30 levels with degree of the chronic HBV infection, we paid attention to the serum CK-18 M30 levels in the CHB patients with severe liver injury and inactive carriers in this meta-analysis. A significantly higher serum CK-18 M30 level was observed in CHB patients with severe liver injury compared to inactive carriers (SMD = 1.29, 95%CI: 0.60 - 1.98, P < 0.001). Sensitivity and publication bias analysis verified the accuracy of this analysis. These results suggest that the serum CK-18 M30 levels are distinguished in the CHB patients with severe liver injury compared to the inactive carriers and healthy controls. Serum CK-18 M30 levels that are determined through non-invasive diagnostic methods could be regarded as a useful biomarker for the diagnosis of chronic hepatitis B.
Balkan et al. reported that the serum CK-18 M30 levels were significantly higher in asymptomatic HBV carriers (198.77 ± 77.62 U/L) than in healthy controls (146.92 ± 40.18 U/L), and the results suggested that 262 U/L cut-off value of CK-18 M30 possessed 85% specificity and 48% sensitivity to distinguish HBeAg-negative CHB from asymptomatic HBV carriers (27). We analyzed the difference of serum CK-18 M30 levels between inactive carriers and healthy controls in this study; a markedly elevated serum CK-18 M30 level was observed in the inactive carriers compared to healthy controls (SMD = 0.63, 95% CI: 0.38 - 0.89, P < 0.001). Sensitivity and publication bias analysis verified the accuracy of this analysis. We tried to interpret the phenomenon with some explanation in our meta-analysis. One possible reason is that most inactive carriers might have liver inflammation actually, while a normal ALT level was observed for more than 6 months during the diagnosis; therefore, the follow-up liver biopsy was quitted. Oliveira et al. carried out an investigation of the liver injury in patients presenting HBsAg(+) for more than six months, Anti-HBe(+)/HBeAg(-), viral load below 2,000 IU/mL, and serum ALT levels less than twice the upper limit of normality. The results showed that 11/27 (40.7%) patients actually had advanced liver injury (35). Besides, research has shown that almost 44% of HBsAg(−) hepatitis patients have a normal range of ALT during the majority period despite having advanced liver diseases (34).
Our study first performed a systematic review of all eligible English publications about the association between serum CK-18 M30 levels and chronic HBV infection. Although the effort was made to conduct a comprehensive analysis, some limitations still should be acknowledged. First, the number of eligible studies and the sample size of the studies were relatively small, which limited the statistical power of our analysis, and might be a main reason for the heterogeneity. Second, although no publication bias was observed in this meta-analysis, the results may not be exact due to the low number of studies. Third, the meta-analysis included English-language studies and grey literature or unpublished studies were not searched. Finally, we did not conduct any meta-regression analysis since the number of included studies was low.
In conclusion, this meta-analysis provides a strong evidence for the association of serum CK-18 M30 levels with the severity of liver injury in chronic hepatitis B patients. The CK-18 M30 levels could be adopted as a useful non-invasive biomarker for the diagnosis of chronic HBV infection. Further studies with larger populations and a comprehensive design are needed to investigate the diagnostic and physiotherapeutic value of CK-18 M30 for chronic hepatitis B patients.