1. Background
Non-alcoholic fatty liver disease (NAFLD) has become the most common cause of liver dysfunction worldwide and is highly prevalent in obese and morbidly obese patients (1, 2). It manifests as the presence of hepatic steatosis in the absence of alcohol-induced liver damage or other causes of liver pathology. It is increasingly associated with diabetes mellitus (DM), hyperlipidemia, and metabolic syndrome (MetS) (3). Its underlying mechanism at the cellular level centers on insulin resistance and an interplay of oxidative stress, lipid peroxidation, cytokines, and adipokines (4). Ensuing deposition of fat leads to liver steatosis and can progress to more severe liver damage in the form of non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and even carcinoma (5). In fact, untreated NAFLD can result in advanced fibrosis in a few years, which per se could significantly increase the mortality risk from coronary heart disease, malignancy, and liver-related problems (6, 7).
Reports on the prevalence of NAFLD have demonstrated that this condition could be present in up to 67% of overweight and 94% of obese patients, and the advanced form of the disease, NASH, was reported in up to 77.5% in a Japanese cohort (8). The prevalence is especially high in patients with morbid obesity because of the accompanied MetS and insulin resistance (9). In a cross-sectional study in the region of the current study, NAFLD was reported to be present in 43.8% of the general population, increasing to up to 70% in patients with MetS (10). However, regional data regarding its prevalence and associated risk factors in people with morbid obesity, as well as the risk of advanced fibrosis is scarce.
Non-invasive diagnosis of NAFLD and liver fibrosis has been the center of attention in recent years and various tools have been suggested to replace liver biopsy (LB) (11). Although liver ultrasound (US) lacks the desired diagnostic accuracy in this regard, it has remained a convenient and accessible method of initial liver evaluation (12). Nevertheless, it is especially incapable of diagnosing fibrosis and can only detect advanced stages when signs of cirrhosis have emerged. As a result, a number of simple tools have recently been validated to predict the risk of liver fibrosis. Among these, NAFLD fibrosis score (NFS) (13) and fibrosis-4 index (FIB-4) (14) have shown the best diagnostic performance for advanced fibrosis and may be used to complement the findings of liver chemistries and US (15). These risk prediction algorithms are based solely on simple blood tests, age, and body mass index (BMI). Nevertheless, while LB has remained the gold standard for the diagnosis of NAFLD and assessment of fibrosis despite having its own risks and shortcomings (16), these noninvasive tools need to be thoroughly studied in various settings before they can be incorporated into everyday practice.
Given the epidemiologic impact of genetic and ethnic variations on NAFLD prevalence (17) and to investigate the diagnostic utility of the aforementioned noninvasive tools, we aimed to evaluate NAFLD and liver fibrosis in a prospectively-maintained database of bariatric patients and study the associated risks and predictive factors.
2. Methods
2.1. Study Population and Design
This study was a baseline evaluation of the Tehran Obesity Treatment Study (TOTS), which is an ongoing prospective bariatric cohort commenced in March 2013. A detailed study protocol for TOTS is available elsewhere (18). Briefly, after providing written informed consent, morbidly obese patients undergo laparoscopic Roux-en-Y gastric bypass, mini-gastric bypass, or sleeve gastrectomy by a single surgical team and are followed postoperatively. A wide range of variables including anthropometric and laboratory indices are collected at baseline, intraoperatively, and postoperatively.
Of the 2007 patients in the database after exclusion of those with a BMI < 35 kg/m2 (n = 11), history of heavy alcohol consumption (defined as average daily pure alcohol consumption of 20 g for females and 30 g for males, or history of past excessive drinking for a period of two years at any time during the past 20 years) (n = 33), seropositivity for hepatitis viruses, or hepatotoxic medication use (n = 5), or age younger than 18 years (n = 14), 1944 participants were selected for the current study.
Preoperative laboratory and anthropometric indices included, but were not limited to, liver function tests (aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), and albumin), lipid profile (high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), total cholesterol level, and serum triglyceride level (TG)), complete blood count, fasting plasma glucose (FPG), serum insulin, and glycosylated hemoglobin (HbA1c) level. US was performed in the immediate preoperative period in all patients by a skilled radiologist to assess liver span and grade fatty liver from 0 to 3, based on the severity of echogenicity.
2.2. Liver Biopsy (LB)
A subset of patients (n = 73) agreed and consented to undergo LB at the time of surgery. Biopsies were taken from the left liver lobe, percutaneously with a 16-gauge Tru-Cut needle (BARD-Max core, Covington, GA, USA), and right liver lobe by wedge biopsy under laparoscopic guidance. This approach ensured that enough tissue would be obtained for evaluation and provided specimens for future molecular and immunohistochemical studies (18). An experienced liver pathologist blinded to baseline characteristics and laboratory data of patients assessed all biopsies using hematoxylin and eosin, Masson’s trichrome, and iron staining. Diagnosis of NAFLD or NASH was made after thorough histologic evaluation of samples. Specimens were evaluated according to NASH-clinical research network’s (NASH-CRN) NAFLD activity score (NAS) criteria (19), which scores three key histologic features of steatosis from 0 to 3, lobular inflammation from 0 to 3, and hepatocyte ballooning from 0 to 2, producing a total score range from zero to eight. Histologic features of fibrosis were also assessed, from F0 (no fibrosis) to F4 (cirrhosis), based on the criteria proposed by Kleiner et al. (19). Other tissue features were also assessed to provide a complete pathology report.
2.3. Definitions
MetS was present if at least three of five criteria according to the Joint Interim Statement (JIS) definition were met (20). High transaminase level was defined as AST levels ≥ 33 U/L or 29 U/L and ALT levels ≥ 43 U/L or 30 U/L in males and females, respectively. Normal liver was defined by either having a normal LB (NAS of 0) or normal liver US (grade 0 fatty liver) in whom LB was not performed; otherwise, patients were categorized as having fatty liver according to LB results (NAS of 1 to 4 as non-alcoholic fatty liver (NAFL), and NAS of 5 to 8 as NASH) or liver US (grade I-III corresponding to mild to severe steatosis). For fibrosis, F2 to F4 was defined as significant fibrosis (SF).
Homeostatic model assessment of insulin resistance index (HOMA-IR) was calculated according to the standard equation and a value above 2.50 mol × µU/L was considered as insulin resistant (IR) (21). NFS was calculated in all NAFLD patients using the formula: -1.675 + 0.037 × age (year) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/DM (present = 1, absent = 0) + 0.99 × AST/ALT ratio - 0.013 × platelet count (109/L) - 0.66 × albumin (g/dL); subjects were categorized as having low, intermediate, or high probability of advanced fibrosis, if they scored less than -1.5, between -1.5 and 0.676, or higher than 0.676, respectively. Moreover, the FIB-4 index was calculated according to the formula: (age (year) × AST (U/L)) / (platelet (109/L) × ALT (U/L)1/2 ), and categorized using cutoffs of 1.45 and 3.25.
2.4. Statistical Analysis
Statistical analyses were performed using SPSS version 21.0 software (SPSS Inc., Chicago, IL, USA). Descriptive statistics were reported as mean ± standard deviation (SD) for continuous variables and number (%) for categorical variables. Variables without a normal distribution were reported as median [25 - 75 interquartile range (IQR)]. Student t-test and analysis of variance (ANOVA) were used to compare normally distributed continuous variables, and Chi-squared test was used for categorical variables. Mann-Whitney and Kruskal-Wallis tests were used for non-normally distributed variables. Chi-squared and Fisher’s exact tests were used to look for associations between different variables. The relationship between the presence of NAFLD as the dependent variable and possible independent predictive variables including age, gender, weight, height, waist circumference (WC), BMI, DM, hypertension (HTN), MetS, ALT, AST, ALP, albumin, HDL, LDL, total cholesterol, TG, FPG, insulin, IR, HbA1c, and HOMA-IR was assessed using binary logistic regression model with the enter method. Those variables with a P value of < 0.2 in the univariate model were selected for multivariate analysis with the backward selection method. Odds ratio (OR) and 95% confidence intervals (95% CI) were calculated and reported. P < 0.05 was considered statistically significant for all comparisons.
Moreover, the association between LB and US for assessing NAFLD was assessed; they were compared as three-level variables (0, grade I to II, and grade III for US versus normal, NAFL, and NASH for LB). Similarly, associations between NFS or FIB-4 and fibrosis (normal liver versus F1-F4) were calculated. Moreover, we calculated the area under the receiver operating characteristic curves (AUROC) of the diagnostic tests, as well as their optimized cutoffs for these diagnostic tests based on AUROC. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated and reported for US, NFS, and FIB-4. Any possible agreements between NFS and FIB-4 was further assessed using the kappa statistic.
2.5. Ethical Considerations
Written informed consent was obtained from all participants before the study, including a separate consent for those undergoing LB. All procedures were performed in accordance with the Helsinki Declaration and its later amendments. The Human Research Review Committee of the Endocrine Research Center, Shahid Beheshti University of Medical Sciences, approved this study (No.2ECRIES 93/03/13).
3. Results
Study participants included 1944 patients with mean age of 38.3 ± 10.8 years, mean BMI of 44.6 ± 6.4 kg/m2, and 79% being female. MetS was present in 63.0%, HTN in 27.4%, and DM in 22.6% of patients, and 75.9% were IR. Median AST and ALT levels were 20 [16 - 26] U/L and 23 [17 - 34] U/L, respectively, and 25.5% of patients had high ALT and 15.7% had high AST levels (Table 1).
| Variable | Total (N = 1944) | Underwent Biopsy (N = 73) |
|---|---|---|
| Age, y | 38.3 ± 10.8 | 40.1 ± 10.9 |
| Sex, female, % | 79 | 72 |
| Weight, kg | 120.7 ± 21.9 | 125.7 ± 19.9 |
| Height, cm | 163.8 ± 9.1 | 165.5 ± 8.9 |
| BMI, kg/m2 | 44.6 ± 6.4 | 45.9 ± 5.6 |
| Waist circumference, cm | 124.2 ± 14.5 | 127.2 ± 13.1 |
| Systolic blood pressure, mmHg | 122.8 ± 12.6 | 124.1 ± 10.6 |
| Diastolic blood pressure, mmHg | 78.0 ± 8.8 | 77.1 ± 7.5 |
| Hypertension | 533 (27.4) | 22 (30.1) |
| Fasting plasma glucose, mg/dL | 98 [90 - 112] | 100 [92 - 116] |
| Insulin, mIU/L | 17.4 [11 - 25] | 18.9 [12 - 25.2] |
| Hemoglobin A1c, % | 5.5 [5.1 - 6.1] | 5.5 [5.1 - 6.0] |
| HOMA-IR | 4.35 [2.66 - 6.78] | 5.02 [3.28 - 6.61] |
| Insulin resistant | 1100 (75.9) | 45 (81.8) |
| Diabetes mellitus, % | 22.6 | 24.3 |
| Metabolic syndrome, % | 63.0 | 68.6 |
| Triglyceride, mg/dL | 142 [104 - 194] | 156 [119 - 200] |
| High-density lipoprotein, mg/dL | 47.7 ± 11.6 | 46.9 ± 10.5 |
| Low-density lipoprotein, mg/dL | 111.5 ± 33.2 | 107.4 ± 34.8 |
| Alkaline phosphatase, IU/L | 188.4 ± 86.1 | 187.9 ± 49.1 |
| AST, U/L | 20 [16 - 26] | 21 [16 - 27] |
| High AST | 306 (15.7) | 13 (17.8) |
| ALT, U/L | 23 [17 - 34] | 24 [18 - 34] |
| High ALT | 496 (25.5) | 20 (27.4) |
| Total cholesterol, mg/dL | 191.8 ± 42.2 | 187.0 ± 43.6 |
| Serum albumin, g/dL | 4.3 ± 0.7 | 4.4 ± 0.3 |
| Platelet count, 103/microL | 281.6 ± 66.3 | 268.3 ± 64.5 |
| Liver span, cm | 15.8 ± 2.2 | 16.7 ± 2.4 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance.
aValues are presented as mean ± SD for normally distributed variables, median [IQR] for not normally distributed variables, or No. (%) for categorical variables.
3.1. Liver Ultrasound
Liver steatosis was present in 76.8% of the patients; 482 (32.3%) had grade I, 664 (44.5%) had grade II, and 347 (23.2%) had grade III fatty liver (Table 2). Significant differences were observed regarding most variables between these groups, with more unfavorable values in higher fatty liver grades. Total cholesterol, LDL, ALP, platelet count, and serum albumin were comparable across groups. Patients with grade-I fatty liver were older and had higher weight, height, WC, HbA1c, and ALT levels than those with normal liver. Those with grade II or III fatty liver had higher values than those with normal or grade I fatty liver for all variables. IR, MetS, and DM were also significantly more prevalent in those with grade III fatty liver, reaching 85.6%, 73.4%, and 34%, respectively.
| Variable | Grade 0 (N = 451) | Grade I (N = 482) | Grade II (N = 664) | Grade III (N = 347) | P Valuec |
|---|---|---|---|---|---|
| Age, y | 36.0 ± 11.2 | 37.7 ± 10.9 # | 39.6 ± 10.3 $, & | 39.5 ± 10.6 $, & | < 0.001 |
| Gender, female, No. (%) | 372 (82.5) | 432 (89.6) | 515 (77.6) | 224 (64.6) | < 0.001 |
| Weight, kg | 118.3 ± 22.4 | 114.0 ± 18.3 # | 121.5 ± 21.1 #, £ | 131.6 ± 22.8 $, £, § | < 0.001 |
| Height, cm | 163.5 ± 8.6 | 161.8 ± 8.3 # | 164.4 ± 9.2 £ | 166.1 ± 9.9 $, £, ¥ | < 0.001 |
| BMI, kg/m2 | 43.8 ± 6.9 | 43.3 ± 5.7 | 44.7 ± 6.0 #, £ | 47.4 ± 6.4 $, £,§ | < 0.001 |
| Waist circumference, cm | 121.8 ± 14.4 | 119.4 ± 13.8 # | 124.9 ± 13.7 $, £ | 132.1 ± 13.8 $, £, § | < 0.001 |
| Systolic blood pressure, mmHg | 121.9 ± 12.4 | 120.5 ± 12.1 | 123.9 ± 12.7 #,£ | 124.9 ± 12.4 $, £ | < 0.001 |
| Diastolic blood pressure, mmHg | 77.0 ± 7.8 | 76.4 ± 8.6 | 79.2 ± 8.9 $, £ | 79.2 ± 9.3 $, £ | < 0.001 |
| Hypertension, No. (%) | 428 (94.9) | 455 (94.4) | 580 (87.3) | 301 (86.7) | < 0.001 |
| Fasting plasma glucose, mg/dL | 94 [86 - 101.2] | 95 [87 - 105] | 101 [92 - 115] $, £ | 104 [94 - 128.7] $, £, ¥ | < 0.001 |
| Insulin, mIU/L | 15.15 [9.30 - 25] | 15.2 [10 - 22.52] | 17.75 [12 - 25.17] #, £ | 21.1 [14.5 - 29.7] $, £, § | < 0.001 |
| Hemoglobin A1c, % | 5.30 [5 - 5.60] | 5.4 [5.1 - 5.9] # | 5.6 [5.2 - 6.2] $, £ | 5.7 [5.2 - 6.5] $, £, ¥ | < 0.001 |
| HOMA-IR | 3.60 [2.18 - 6.05] | 3.63 [2.31 - 5.73] | 4.64 [3.08 - 7.11] $, £ | 5.57 [3.81 - 8.2] $, £, § | < 0.001 |
| Insulin resistant, No. (%) | 194 ( 66.9) | 257 (68.9) | 423 (81.0) | 226 (85.6) | < 0.001 |
| Diabetes mellitus, No. (%) | 44 (12.1) | 72 (15.9) | 175 (27.3) | 115 (34) | < 0.001 |
| Metabolic syndrome, No. (%) | 174 (47.4) | 252 (55.3) | 462 (71.9) | 248 (73.4) | < 0.001 |
| Triglyceride, mg/dL | 128 [94 - 175] | 127 [97.5 - 170] | 151 [110 - 203] $,£ | 157.5 [123 - 206] $, £, ¥ | < 0.001 |
| High-density lipoprotein, mg/dL | 48.8 ± 11.5 | 48.4 ± 11.5 | 47.4 ± 11.3 | 46.0 ± 12.2 #, & | 0.008 |
| Low-density lipoprotein, mg/dL | 113.2 ± 30.6 | 112.1 ± 32.4 | 111.8 ± 34.6 | 108.4 ± 34.3 | 0.271 |
| Alkaline phosphatase, IU/L | 188.4 ± 63.1 | 190.7 ± 103.8 | 186.4 ± 60.6 | 189.2 ± 116.7 | 0.882 |
| AST, U/L | 18 [14 - 25] | 18 [15 - 24] | 20 [16 - 27] $, £ | 23 [17 - 33] $, £, § | < 0.001 |
| High AST, No. (%)d | 38 (8.4) | 52 (10.8) | 124 (18.7) | 92 (26.5) | < 0.001 |
| ALT, U/L | 20 [15 - 28] | 21 [16 - 30] # | 25 [18 - 37] $, £ | 28 [20 - 45.25] $, £, ¥ | < 0.001 |
| High ALT, No. (%)d | 63 (14.0) | 103 (21.4) | 205 (30.9) | 125 (36.0) | < 0.001 |
| Total cholesterol, mg/dL | 193.0 ± 40.8 | 190.4 ± 41.2 | 192.8 ± 43.9 | 190.3 ± 41.7 | 0.670 |
| Serum albumin, g/dL | 4.30 ± 0.34 | 4.29 ± 0.35 | 4.34 ± 0.37 | 4.32 ± 0.41 | 0.312 |
| Platelet count, 103/microL | 279.3 ± 64.0 | 285.8 ± 65.7 | 282.2 ± 65.6 | 277.3 ± 70.8 | 0.305 |
| Liver span, cm | 14.7 ± 2.2 | 15.1 ± 2.0 | 15.8 ± 2.1 #, & | 16.4 ± 2.3 $, £, ¥ | < 0.001 |
Abbreviations: BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance index; AST, aspartate transaminase; ALT, alanine transaminase.
aValues are presented as mean±SD for normally-distributed variables, median [IQR] for not normally-distributed variables, and n (%) for categorical variables.
b#/$ = <0.05/<0.001 vs. normal; &/£ = <0.05/<0.001 vs. grade I; ¥/§ = <0.05/<0.001 vs. grade II
cNormally-distributed variables were analyzed using t-test and ANOVA, and variables with other distributions were analyzed using Mann-Whitney and Kruskal-Wallis tests.
dHigh transaminase level was defined as AST levels! 33 U/L or 29 U/L and ALT levels! 43 U/L or 30 U/L in men and women, respectively.
Logistic regression analysis revealed that DM (OR 2.46, 95% CI 1.75 to 3.44), MetS (OR 2.24, 95% CI 1.78 to 2.83), and HTN (OR 1.78, 95%CI 1.36 to 2.34) increased the odds of having fatty liver. Other factors associated with the presence of fatty liver included age, weight, BMI, WC, FPG, ,HbA1c, IR, HOMA-IR, high AST, high ALT, diastolic blood pressure, and TG. On multivariate analysis, only Mets (OR 1.70, 95%CI 1.15 to 2.50), HbA1c (OR 1.345, 95% CI 1.08 to 1.67), age (OR 1.026, 95% CI 1.01 to 1.04), and high ALT (OR 1.02, 95%CI 1.01 to 1.04) were associated with higher chance of NAFLD, and systolic blood pressure was inversely related to NAFLD (OR 0.97, 95% CI 0.96 to 0.99, Table 3).
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |
| Diabetes mellitus | 2.460 | 1.757 - 3.446 | < 0.001 | |||
| Metabolic syndrome | 2.246 | 1.780 - 2.834 | < 0.001 | 1.701 | 1.157 - 2.500 | 0.007 |
| Hypertension | 1.789 | 1.363 - 2.347 | < 0.001 | |||
| Insulin resistance | 1.772 | 1.337 - 2.348 | < 0.001 | |||
| HbA1c | 1.543 | 1.336 - 1.781 | < 0.001 | 1.345 | 1.078 - 1.679 | 0.009 |
| HOMA-IR | 1.040 | 1.007 - 1.074 | 0.018 | |||
| High ASTa | 1.033 | 1.019 - 1.046 | < 0.001 | |||
| Age | 1.027 | 1.017 - 1.038 | < 0.001 | 1.026 | 1.009 - 1.044 | 0.003 |
| High ALTa | 1.026 | 1.017 - 1.036 | < 0.001 | 1.020 | 1.001 - 1.038 | 0.035 |
| BMI | 1.026 | 1.009 - 1.044 | 0.003 | |||
| DBP | 1.017 | 1.004 - 1.029 | 0.011 | |||
| WC | 1.015 | 1.006 - 1.023 | 0.001 | |||
| FPG | 1.013 | 1.008 - 1.018 | < 0.001 | |||
| Weight | 1.007 | 1.002 - 1.012 | 0.008 | |||
| TG | 1.004 | 1.002 - 1.006 | < 0.001 | |||
| SBP | 1.007 | 0.998 - 1.017 | 0.113 | 0.978 | 0.963 - 0.993 | 0.005 |
Abbreviations: HOMA-IR, homeostatic model assessment of insulin resistance index; AST, aspartate transaminase; ALT, alanine transaminase; BMI, body mass index; DBP, diastolic blood pressure; WC, waist circumference; FPG, fasting plasma glucose; TG, triglyceride; SBP, systolic blood pressure.
aHigh transaminase level was defined as AST levels ≥ 33 U/L or 29 U/L and ALT levels ≥ 43 U/L or 30 U/L in men and women, respectively.
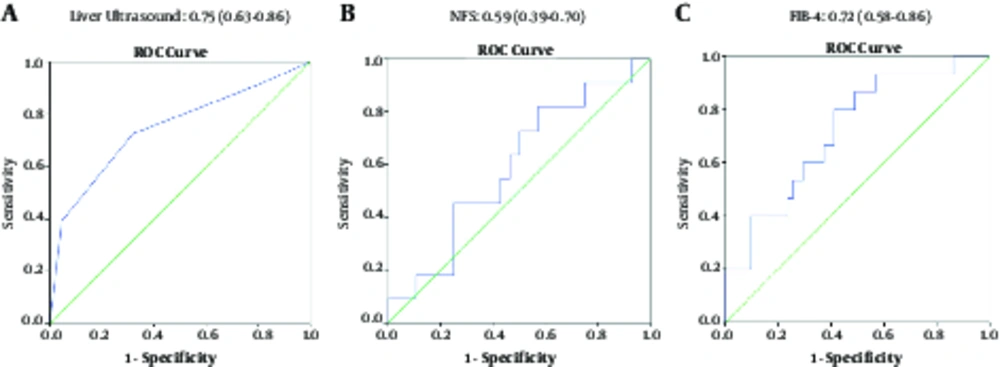
Fatty liver grade on US showed a significant association (P < 0.001) with LB. The AUROC of US for diagnosing NAFLD was 0.75 (95% CI 0.63 - 0.86, P = 0.001, Figure 1). At the cutoff of grade I, US yielded a sensitivity of 90.2%, PPV of 73%, specificity of 22.7%, and NPV of 50% for diagnosing NAFLD. However, it failed to show any association with NASH at this cut-off (P = 0.583). At cutoff of grade II fatty liver, US yielded a sensitivity of 72.5%, PPV of 84.1%, specificity of 68.2%, and NPV of 51.7% for diagnosis of NAFLD (P = 0.002) and sensitivity of 100%, PPV of 15.9%, specificity of 43.9%, and NPV of 100% for diagnosis of NASH (P = 0.024, Table 4).
| Diagnostic Test | Cutoff | Diagnosis | AUROC (Range) | P Value | Sensitivity, % | Specificity, % | Positive Predictive Value, % | Negative Predictive Value, % |
|---|---|---|---|---|---|---|---|---|
| Liver ultrasound | Grade 1 | NAFL | 0.75 (0.63 - 0.86) | 0.001 | 90.2 | 22.7 | 73 | 50 |
| Grade 2 | NAFL | - | 0.002 | 72.5 | 68.2 | 84.1 | 51.7 | |
| Grade 2 | NASH | - | 0.024 | 100 | 43.9 | 15.9 | 100 | |
| NFS | -2.5 | Fibrosis | 0.59 (0.39 - 0.70) | 0.382 | 90.9 | 25 | 32.2 | 87.5 |
| FIB-4 | 0.5 | Fibrosis | 0.72 (0.58 - 0.86) | 0.010 | 93.3 | 43.1 | 32.5 | 95.6 |
Abbreviations: AUROC, area under the receiver operating characteristic curve; NAFL, non-alcoholic fatty liver; NASH, non-alcoholic steatohepatitis; NFS, NAFLD fibrosis score; FIB-4, Fibrosis-4 index.
aWe calculated optimized cutoffs for liver ultrasound (for diagnosis of NAFL/NASH according to biopsy) and for NFS and FIB-4 (for diagnosis of fibrosis according to biopsy)
3.2. Liver Biopsy
Seventy-three patients underwent LB. Their mean BMI was 45.9 ± 5.6 kg/m2 with a mean age of 40.1 ± 10.9 years with 72% being female. MetS was present in 68.6%, HTN in 30.1%, and DM in 24.3% of this patient subset. Patients with NAFL or NASH were significantly older and had higher AST and ALT levels than those with normal liver. However, no difference was observed regarding all other variables, including lipid profile and anthropometrics (Table 5).
| Variable | Normal | NAFL / NASH | ||
|---|---|---|---|---|
| NAS = 0 (N = 22) | 1 ≤ NAS ≤ 4 (N = 44) | 5 ≤ NAS ≤ 8 (N = 7) | P Valueb | |
| Age, y | 34.0 ± 10.0 | 43.5 ± 10.2 | 37.5 ± 10.5 | 0.002 |
| Sex, female | 18 (81.8) | 32 (72.7) | 3 (42.9) | 0.128 |
| Weight, kg | 123.8 ± 20.5 | 124.3 ± 19.6 | 140.5 ± 16.4 | 0.601 |
| Height, cm | 164.5 ± 9.0 | 165.2 ± 8.8 | 170.5 ± 8.5 | 0.534 |
| BMI, kg/m2 | 46.1 ± 6.4 | 45.4 ± 5.2 | 48.2 ± 5.9 | 0.845 |
| Waist circumference, cm | 123.9 ± 14.9 | 127.9 ± 11.9 | 133.5 ± 12.1 | 0.155 |
| Systolic blood pressure, mmHg | 121.8 ± 8.2 | 125.0 ± 10.2 | 126.4 ± 18.4 | 0.216 |
| Diastolic blood pressure, mmHg | 75.9 ± 7.3 | 77.2 ± 7.2 | 80.0 ± 10.0 | 0.370 |
| Hypertension | 22 (100) | 40 (90.9) | 5 (71.4) | 0.057 |
| Fasting plasma glucose, mg/dL | 97.5 [90.25 - 106] | 102 [91 - 121] | 98 [98 - 123] | 0.289 |
| Insulin, mIU/L | 18.72 [8.82 - 24.85] | 18 [11.48 - 24.7] | 27.33 [23.32 - 43.87] | 0.725 |
| Hemoglobin A1c, % | 5.5 [5.12 - 6.10] | 5.50 [5.15 - 6.10] | 5.50 [5.10 - 5.60] | 0.259 |
| HOMA-IR | 4.92 [3.45 - 6.94] | 4.36 [3.22 - 6.23] | 8.26 [5.60 - 12.88] | 0.517 |
| Insulin resistant | 12 (75) | 27 (81.8) | 6 (100) | 0.597 |
| Diabetes mellitus | 3 (15) | 12 (27.9) | 2 (28.6) | 0.577 |
| Metabolic syndrome | 11 (55) | 31 (72) | 6 (85.7) | 0.265 |
| Triglyceride, mg/dL | 147 (101 - 173) | 170.2 ± 83.3 | 193.5 ± 86.2 | 0.367 |
| High-density lipoprotein, mg/dL | 46.2 ± 11.2 | 47.9 ± 10.7 | 43.2 ± 6.6 | 0.716 |
| Low-density lipoprotein, mg/dL | 104.9 ± 33.5 | 109.0 ± 35.7 | 105.4 ± 38.0 | 0.707 |
| Alkaline phosphatase, IU/L | 191.2 ± 50.4 | 190.5 ± 48.6 | 162.7 ± 47.8 | 0.727 |
| AST, U/L | 16.5 [14.25 - 20.25] | 21 [18 - 26.5] | 33 [27 - 36] | 0.002 |
| Elevated AST | 1 (4.5) | 8 (61.5) | 4 (57.1) | 0.011 |
| ALT, U/L | 18.5 [15.5 - 22.75] | 27.65 [19.75 - 35.25] | 37 [33.5 - 56] | < 0.001 |
| Elevated ALT | 1 (4.5) | 14 (31.8) | 5 (71.4) | < 0.001 |
| Total cholesterol, mg/dL | 185.3 ± 34.9 | 187.8 ± 47.8 | 186.8 ± 44.7 | 0.841 |
| Serum albumin, g/dL | 4.4 ± .3 | 4.5 ± 0.4 | 4.3 ± 0.2 | 0.812 |
| Platelet count, 103/microL | 280.1 ± 57.1 | 268.6 ± 66.4 | 226.8 ± 68.2 | 0.333 |
| Liver Ultrasound | 0.333 | |||
| Normal (steatosis grade 0) | 5 (50) | 5 (50) | 0 | |
| NAFLD (steatosis grades I - III) | 17 (27) | 39 (62) | 7 (11) | |
Abbreviations: BMI, body mass index; HOMA-IR, homeostatic model assessment insulin resistance index; AST, aspartate transaminase; ALT, alanine transaminase.
aValues are presented as mean±SD for normally-distributed variables, median [IQR] for not normally-distributed variables, and n (%) for categorical variables.
bFor statistical analysis, comparison was performed between two groups of normal (NAS = 0) vs. NAFLD (NAS ≥ 1) to increase statistical power, using parametric or non-parametric tests where appropriate.
Biopsy results demonstrated that nearly 70% of patients had features of NAFLD: 60.3% had NAFL and 9.6% had NASH. Median NAS was 2 [0 - 4]. The majority of patients (82%) had < 33% steatosis, 87% had < 2 foci/200x lobular inflammation, and 87% had no or few balloon cells in their biopsies (Table 6). Among other common biopsy features were the presence of acidophil bodies (95.9%), microgranulomas (92%), and megamitochondria (97.3%). Fibrosis features were detected in 17 (23%) patients: 14 patients (19%) had F1 and three patients had F2, F3, and F4, each.
| Item | Extent | Score | Prevalence, % |
|---|---|---|---|
| NAS | |||
| Steatosis, % | |||
| Gradea | < 5 | 0 | 41.1 |
| 5 - 33 | 1 | 41.1 | |
| > 33 - 66 | 2 | 16.4 | |
| > 66 | 3 | 1.4 | |
| Microvesicular steatosis | 63 | ||
| Inflammation | |||
| Lobular inflammationa | No foci | 0 | 38.4 |
| < 2 foci/200x | 1 | 49.3 | |
| 2 - 4 foci/200x | 2 | 12.3 | |
| > 4 foci/200x | 3 | 0 | |
| Microgranulomas, % | 92 | ||
| Large lipogranulomas, % | 78 | ||
| Portal inflammation, % | 63 | ||
| Liver cell injury | |||
| Ballooninga | None | 0 | 43.8 |
| Few balloon cells | 1 | 43.8 | |
| Many cells/prominent ballooning | 2 | 12.3 | |
| Acidophil bodies | 95.9 | ||
| Pigmented macrophages | 74 | ||
| Megamitochondria | 97.3 | ||
| Other findings | |||
| Mallory’s hyaline | 94.4 | ||
| Glycogenated nuclei | 60.5 | ||
| Iron deposition | 88.7 | ||
| Total NAS score | Normal | 0 | 30.1 |
| NAFL | 1 - 4 | 60.3 | |
| NASH | 5 - 8 | 9.6 | |
| Fibrosis stage | |||
| None | Normal | 76.7 | |
| Perisinusoidal or periportal | F1 | 19.2 | |
| Perisinusoidal or portal/periportal | F2 | 1.4 | |
| Bridging fibrosis | F3 | 1.4 | |
| Cirrhosis | F4 | 1.4 |
Abbreviations: NAS, non-alcoholic fatty liver disease activity score; NAFL, non-alcoholic fatty liver, NASH, non-alcoholic steatohepatitis.
aFeature scores added to calculate NAS.
3.3. NAFLD Fibrosis Score (NFS) and Fibrosis-4 Index (FIB-4)
NFS was calculated in 1077 patients. Four hundred and forty-five (41.3%) patients had a low risk of fibrosis, compared to 520 (48.3%) with moderate and 112 (10.4%) with high risk of advanced fibrosis (Table 6). NFS was significantly associated with fibrosis (P = 0.042) and SF (P = 0.013). However, AUROC failed to show statistical significance for diagnosis of fibrosis or SF (Table 4, Figure 1). Its sensitivity, specificity, PPV, and NPV for diagnosing fibrosis were 90.9%, 25%, 32.2%, and 87.5%, respectively (Table 4). NFS was also associated with NAFLD on US, and higher fatty liver grades were associated with higher NFS and hence, higher fibrosis risk (P < 0.001, Table 2).
Fibrosis-4 Index was calculated in 1699 patients. Overall, 1636 patients (96.2%) had a score below the cutoff of 1.45, 62 (3.2%) between 1.45 and 3.25, and only one patient higher than 3.25. FIB-4 showed a significant association with the presence of fibrosis and SF (P = 0.01 and P < 0.001, respectively). AUROC of FIB-4 for diagnosing fibrosis was 0.72 (95% CI 0.58 - 0.86, P = 0.01) with a sensitivity of 93.3%, specificity of 43.1%, PPV of 32.5% and NPV of 95.6% at cutoff of 0.5 (Table 4, Figure 1). Moreover, three diagnostic categories of FIB-4 and NFS showed a significant association (P < 0.001); however, they had minimal agreement (kappa = 0.022, P = 0.028).
4. Discussion
This study demonstrated the high prevalence of NAFLD in our bariatric patients at baseline, in up to 76% of patients according to US, and in 70% of those undergoing LB. In this latter group, 10% had NASH and 23% had histologic features of fibrosis. This high prevalence is significant when considered together with the strong association that was found between the presence of NAFLD, and DM, MetS, and IR, which themselves are on the rise in our country (22).
A recent meta-analysis estimated the global prevalence of NAFLD in the general population to be around 25%, with the highest values observed in the Middle East region, reaching 31% (23). However, in Iran, a population-based study of 5023 individuals in 2014 yielded an alarming prevalence of 43.8%, much higher than such estimate (10). When DM, dyslipidemia, obesity, and MetS are added to the clinical picture, the overall prevalence increases dramatically to 70% and higher (5, 10, 24). In the context of severe obesity, a benchmark study of 1000 morbidly obese patients undergoing bariatric surgery revealed NAFLD prevalence of 80.2%, consisting of 65.9% with simple steatosis and 14.3% with NASH (25). Our results closely compared to these findings and confirmed the exceptionally high prevalence of the disease in morbidly obese patients, both by US and LB. As the increased risk of liver-specific and overall mortality associated with the severe form of the disease, NASH, is well established (26, 27), these findings call for timely prevention and management of patients to prevent NAFL progression towards NASH and liver fibrosis.
Many studies have investigated various clinical and para-clinical parameters and their association with NAFLD. These include patient’s age, WC, BMI, HTN, DM, dyslipidemia, and high serum ALT, AST, ALP, gamma glutamyl transferase (GGT), FPG, and HOMA-IR (9, 11, 28-32). Other novel markers such as hepatic leptin receptor down-regulation (33), serum alpha-ketoglutarate levels (34), and most recently, serum cytokeratin-18 levels (35) have also been suggested. In line with and complementary to these findings, the current study showed that older age and higher ALT and AST levels are associated with higher NAS on LB. Moreover, HTN, DM, MetS, IR and higher weight, BMI, WC, diastolic blood pressure, AST, ALT, FPG, HbA1c, TG, and HOMA-IR levels were risk factors for NAFLD. While these parameters may be of limited predictive value individually since they are inconsistently associated with NAFL/NASH across studies, the cumulative presence of these derangements can provide a more reliable clue to an underlying NAFLD. As such, DM, MetS, dyslipidemia, and obesity may be the more appropriate and broader entities to look for when determining the risk of NAFLD (36). They may thus warrant further evaluation of patients for NAFLD and related comorbidities.
Developing alternative, noninvasive methods for diagnosing NAFLD has attracted significant interest in recent years. US has always been a simple, feasible, and accessible method for liver assessment. However, it has mostly failed to prove reliable and accurate for NAFLD, especially at higher levels of steatosis (37) or for distinguishing NAFL and NASH (38). Its lack of accuracy for fibrosis has also been another shortcoming (39). US demonstrated a sensitivity of 90% or 72.5% and specificity of 22% or 68% at the cutoff of grade I or II fatty liver, respectively, alongside a significant association with NAS in the current report. This suboptimal performance may be explained by lack of NAFLD-characteristic findings and interference of abdominal wall fat in morbidly obese patients with US imaging (40). However, US demonstrated a fair to good AUROC for diagnosing NAFLD and thus defends its role as a suitable primary work-up method. Other noninvasive methods for diagnosis of NAFLD include fatty liver index (FLI) and United States FLI (USFLI), which have been used and validated by a number of studies (36, 41). They take into account the ethnicity of the patient, which may provide a more individualized approach and prove to be a generalizable tool. Unfortunately however, the researchers were unable to use and compare these tools in this study since GGT data was not available.
For noninvasive diagnosis of fibrosis, both NFS and FIB-4 have been endorsed by the American Association for the Study of Liver Diseases (AASLD) (36). NFS has shown a sensitivity of 66.8% and specificity of 87.5% for detecting SF (15). In the current study, however, AUROC of NFS failed to show significance, presumably due to the fact that it has been validated for detection of advanced fibrosis (F3 - F4), although it has also been used for other definitions of fibrosis, such as SF (15). However, the relatively small number of patients undergoing LB with only a few patients with high-stage fibrosis precluded the researchers from investigating NFS’s accuracy for detecting advanced fibrosis. Nevertheless, a positive correlation was observed between NFS and fibrosis on LB, and although at a lower threshold, yielded acceptable performance. We also found that fatty liver grade on US was associated with higher NFS. On the other hand, FIB-4 showed an AUROC of 0.72, as well as significant association with fibrosis on LB. In line with an AUROC of 0.73 for diagnosing SF in patients with NAFLD (15), the current report showed that FIB-4 had similar accuracy for detecting fibrosis at a lower threshold. At its suggested thresholds, FIB-4 has shown a sensitivity of 64.8% and specificity of 72.9% (15). However, for diagnosing fibrosis, its optimal threshold was 0.5, corresponding to the sensitivity and specificity of 93% and 43%, respectively. The FIB-4 may thus be used for diagnosis of fibrosis as well. If consistently confirmed in larger studies, a primary finding of steatosis on US combined with a high NFS or FIB-4 in the context of other high-risk conditions (i.e. MetS, DM and IR) might be used to detect LB candidates.
Although NFS and FIB-4 showed a significant association in this study, their agreement for diagnosing fibrosis was minimal. Besides from the suboptimal power of our study to compare their performance for diagnosis of advanced fibrosis, this might be attributable to their variable utility in patients with various degrees of fibrosis. Despite the high prevalence of NAFLD, fibrosis was uncommon in our morbidly obese patients and seen only in mild stages. Thus NFS, which takes into account both the BMI and IR, tended to be higher than FIB-4, which is only based on age, AST, ALT, and platelets. As a result, NFS overestimated fibrosis, let alone SF or advanced fibrosis, while FIB-4 showed better clinical utility. This provides an interesting perspective which needs to be further investigated in more comprehensive studies. Another factor to take into account is ethnicity, which was shown to influence the accuracy of these noninvasive tests that were mostly obtained from studies in white populations (42); this may in turn undermine their generalizability.
Finally, although LB is the gold standard and most accurate method for NAFLD diagnosis, it is not always feasible or justified in all bariatric patients due to its associated morbidity and very rare mortality risk. On the other hand, even when LB is performed, interpretation of its results would be subject to sampling error and inter-observer variability. There are also limitations in the use of the NAS system and a cut point of five for diagnosing NASH, as demonstrated in a study by Chalasani et al. (36), in which only 75% of patients with definite histologic diagnosis of NASH had a NAS ≥ 5; this may lead to overlooking a subset of NASH patients, who scored lower than 5. Nevertheless, LB is still the most accurate and reliable method of evaluating NAFLD and fibrosis (36). In light of the current findings, the authors believe that careful stratification of patients at baseline by using universal and non-invasive diagnostic tools, such as liver enzyme levels, US, NFS, and FIB-4 would identify patients who might further benefit from LB to confirm the diagnosis and guide the treatment.
Despite being among the first reports in this region, the current study had a number of limitations. Because of the lack of data on GGT, we were not able to calculate FLI or USFLI, which otherwise would have provided an interesting comparison alongside liver US and LB results. In addition, although many methods were incorporated to minimize missing data, NFS could be calculated in about 72% and FIB-4 in 87% of patients with NAFLD, which is far from ideal. The relatively small number of patients undergoing LB restricted performing more robust analysis (including sensitivity analysis) and comparisons across different diagnostic tools. Lastly, only one pathologist interpreted LB results due to our limited resources.
In conclusion, this study demonstrated a high prevalence of NAFLD but low prevalence of fibrosis in our bariatric population. Diabetes mellitus and metabolic syndrome remain the strongest predictive factors for the presence of NAFLD and NASH and the importance of immediate action for their effective prevention and diagnosis cannot be overemphasized, given the growing pandemic of obesity in the Iranian population and around the world. This study further evaluated the clinical utility of US, NFS, and FIB-4, and demonstrated that while they can have specific uses in practice, they have questionable accuracies and association with biopsy findings and may fall short of replacing LB in certain populations, those with mild stages of fibrosis. Future follow-up studies of our patients will further shed light on other aspects of this condition, including its treatment and prognosis in the short and long term.