1. Context
Currently, hepatitis B remains a health problem all over the world, as it can potentially affect more than 2 billion people worldwide (1). Hepatitis B infection, especially in cases with the chronic form, is associated with severe conditions, such as cirrhosis and hepatocellular carcinoma (HCC) (2). The most susceptible groups to develop the chronic form of hepatitis B are infants and children under five years old (3). As hepatitis B virus persists in body fluids, the main transmission routs are mother to new born transmission, sexual intercourse, IV drug administration, and occupational contact (4). According to the latest meta-analysis of HBV infection, prevalence in Iran varies from 0.87% to 8.86% in different provinces (5). About 5% of infected individuals will remain as chronic carriers and thus vaccination against HBV is an efficient way to stop spreading of infection (6). In order to prevent HBV infection in 1991, the world health organization (WHO) asked its members to start a national vaccination program, especially in endemic regions (7). In Iran, an infant vaccination program was started in 1993 and it is claimed that over 98% of infants are covered (8). Vaccination in children could prevent both acute and chronic forms of infection and reduce complications of HBV infection. Vaccination with HBV subunit vaccine is correlated with a protective level of humoral immunity in 90% of vaccinated cases, yet the titer of antibody could decline over time (9). Anti-HBs antibody titration is considered as a means to evaluate protection in vaccinated individuals. Cases with more than 10 mIU/mL of anti-HBs antibody are assumed as protected (10). The persistence of immunity against HBV in vaccinated individuals depends on immunological memory and high antibody titer (11). Despite high efficacy of vaccination against HBV in Iran (67% to 100% efficacy), some factors such as age, gender, vaccination dosage etc., seem to affect the vaccination efficacy (12). Also it has been documented that some cases constitutively show no response to HBV vaccination (13). Specially in children, a lack of response to HBV vaccine has been shown, which may be due to immune deficiencies, genetic disorders or improper vaccination procedures (14). According to other meta-analysis studies in Iran, the efficacy of hepatitis vaccination varies in different ethnic groups and different subjected sub-populations. A recent meta-analysis study reported 86.3% vaccination efficacy in the general population yet 59.6% in specific patient subpopulations (15). Another meta-analysis showed 93.1% protection among health staff, six month after vaccination (12). Although several meta-analysis studies have investigated HBV vaccination program efficacy in different populations in Iran, yet none of them exclusively surveyed HBV vaccination efficacy in under five-year-old children (8, 12, 15). Regarding the mentioned data about the importance of protective immune response against HBV in infants and young children and considering the possibility of lower immune response in this population, this study performed a systematic review and meta-analysis to investigate HBV vaccination protectivity and efficiency in children under five years age in Iran and summarized the present data on this issue.
2. Objective
A systematic review and meta-analysis was carried out using published articles associated with evaluation of seroconversion rates after hepatitis-B vaccination efficacy in under five-year-old children in Iran.
3. Data Sources
All databases including SID, Magiran, Iranmedex Irandoc, Medlib, PubMed, Medline, and ISI from 2000 to 2017, were searched using keywords and all possible combinations: Hepatitis B, vaccination, efficacy, children, and Iran. Also, titles from the search and references in the selected articles were used as additional search tools.
4. Study Selection
The selection of articles for the study was based on three stages, title, abstract, and full text. Studies with inadequate information, review studies, congresses abstracts, studies published in languages other than English and Persian, systematic studies, meta-analysis, and repeated studies were excluded from the analysis.
The main criterion for Inclusion of different articles in this research was Reference to the efficacy of the hepatitis-B vaccine in under five-year-old children in Iran.
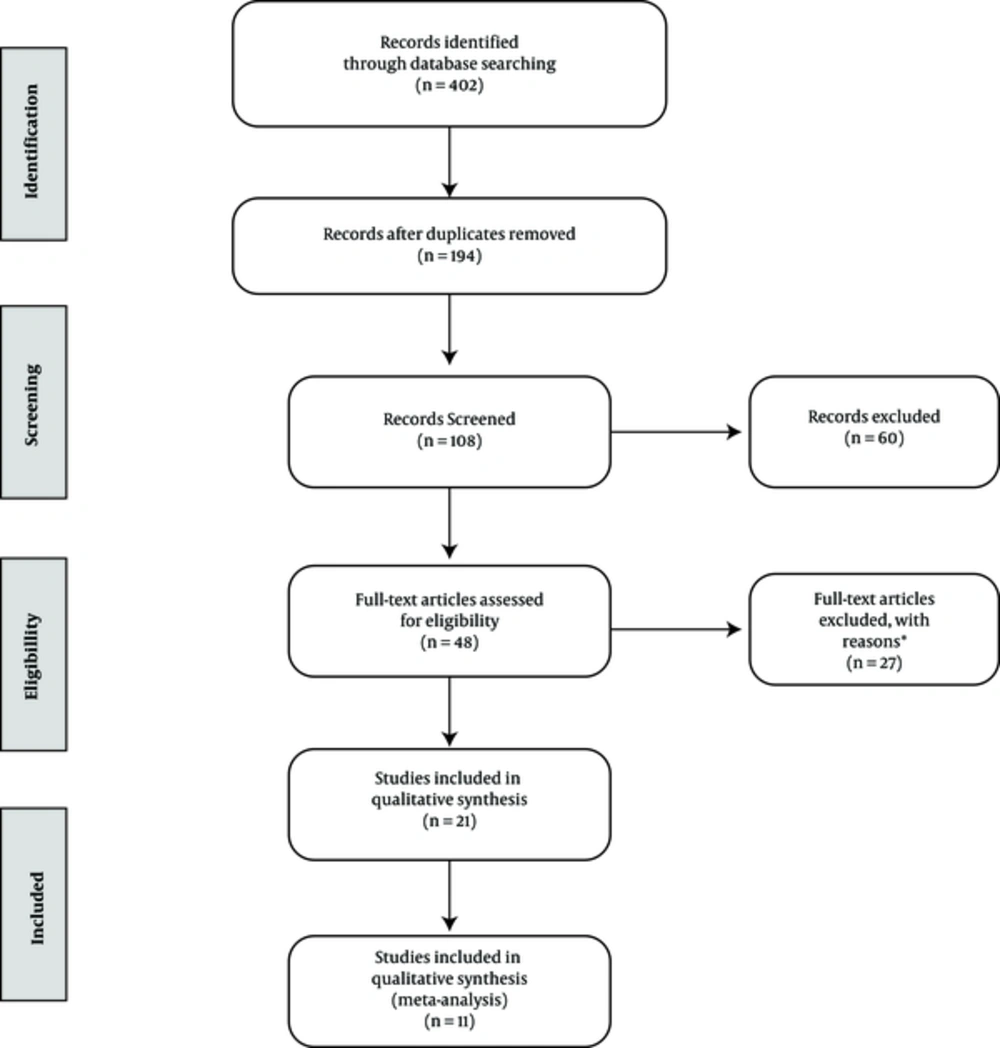
The exclusion criteria of studies included no mention of efficacy of hepatitis-B vaccine, sample with age of over five years old, review studies, and repeated studies. The researchers initially collected all articles related to the efficacy of hepatitis-B vaccine in Iranian children. Then, a checklist of abstracts was prepared. In this phase, 142 articles, which mentioned in their title “The efficacy of hepatitis B vaccine in Iran”, were included in the initial list. Subsequently, each single article was independently studied, 22 of them were removed due to duplication, 42 articles were excluded as their subjects were over five years old, 14 articles due to the inaccessibility of full text, and 17 meta-analysis and review articles were also removed (Figure 1).
5. Data Extraction
After selecting high-quality articles, 11 publications were examined by two reviewers, independently, using a checklist of information that was designed for data extraction.
Finally, for the selected articles, the following data, first author, year of publication, place of study, sample size, age, gender, type of vaccine and percentage of efficacy of hepatitis-B vaccine, was extracted (Table 1).
| Authors Name | Years | Location | Sample Size | Age | Type of Vaccine | Dose of Vaccine, µg | Vaccine Efficacy AntiHBS > 10, mIU/mL, % | Mean of HBsAb, mIU/mL |
|---|---|---|---|---|---|---|---|---|
| Moradi A (14) | 2008 | Gorgan | 215 | 7 - 12, mo | Euvax B, aKorean | 86 | 173.5 | |
| Ahmadi M (16) | 2012 | Bandarabbas | 92 | 12 - 15, mo | NA | 95.66 | 231 | |
| Ahmadi M (16) | 2012 | Bandarabbas | 94 | 21 - 24, mo | NA | 78.2 | 142.9 | |
| Azarkar Z (6) | 2004 | Mashad | 100 | 12 - 16, mo | Eberbiovac | 81 | ||
| Salehi M (17) | 2002 | Zahedan | 324 | 15 - 23, mo | Herberbiovac | 78.4 | ||
| Shokri F (18) | 2001 | Kerman | 735 | Neonate | Herberbiovac | 10 | 95.2 | 302 |
| Rostami N (19) | 2006 | Orumie | 97 | 15, mo | Heberbiovac | 10 | 85.6 | |
| Rezaei M (20) | 2014 | Semnan | 67 | < 5, y | Cuban Vaccine | 87 | ||
| Hosseini MJ (21) | 2009 | Tehran | 165 | 1.5 - 5 | NA | 91 | 232.64 | |
| Jafarzadeh A (22) | 2004 | Urmia | 290 | Neonate | Herberbiovac | 10 | 98.3 | |
| Jafarzadeh A (22) | 2004 | Kerman | 231 | Neonate | Herberbiovac | 10 | 96.1 | |
| Zamani A (23) | 2001 | Tehran | 115 | 12 - 24, mo | Heber Biotec | 10 | 94.8 | |
| Dahifar H (24) | 2004 | Tehran | 538 | 15 - 45, mo | Herberbiovac | 10 | 84.4 | 294 |
| Total | 3063 |
The Characteristics of Different Articles Related to the Efficacy of Hepatitis-B Vaccine Efficacy in Under Five-Year-Old Children in Iran
Statistical analyses for particular factors were performed using the STATA software version 11.2 (STATA Corp LP, College Station, TX, USA) for Windows. The variance of each study was calculated using the binomial distribution formula. Statistical heterogeneity between studies was assessed using Moran’s I2 and Cochran’s Q-tests (at alpha/significance level < 10%). Meta-regression was used to estimate the efficacy of hepatitis-B vaccine efficacy in under five-year-old children in Iran. The results of studies were combined using the random-effects meta-analysis model. A P value of less than 0.05 for heterogeneity tests was considered significant.
6. Results
In the present study, 11 publications (11 studies) were included in the review. Figure 2 shows a flowchart of the search strategy and study selection, along with the reasons for exclusions. In total, 3063 children (1592 males and 1471 females) were examined in these studies.
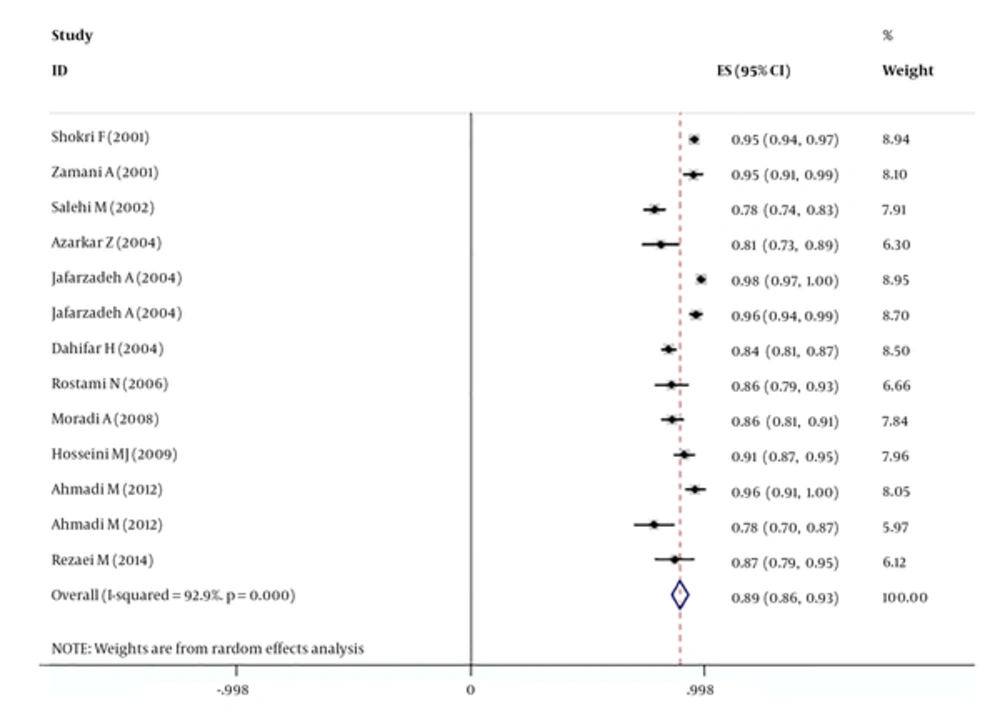
The results showed that hepatitis-B vaccine efficacy in under five-year-old children in Iran was 89% (95% CI: 86% to 93%) with high heterogeneity (P value for heterogeneity, < 0.001, I2 = 92.9).
The highest and lowest hepatitis-B vaccine efficacy was observed in the study of Jafarzadeh et al. in Uromie (northwest of Iran) and Ahmadi et al. in Bandarabbas (south of Iran) in that efficacy was 98.3% (95% CI: 97 to 100) and 78.2% (95% CI: 70 to 87), respectively (Table 1).
The efficacy of the hepatitis-B vaccine in males and females was 85% (95% CI: 78 to 91) and 88% (95% CI: 83 to 93), respectively.
General information about selected papers in this study is listed in Table 1. Figure 1 shows the efficacy of the hepatitis-B vaccine in under five-year-old children in Iran, based on the random effects model.
7. Conclusions
Despite national and international vaccination programs, HBV infection is still a global health concern with certain morbidity (25). One of the most susceptible populations to be effected by HBV and to develop chronic form of HBV infection is under five-year-old children (26). The current meta-analysis is the first to study the efficacy of HBV vaccination, specifically in the under five-year-old population. This study investigated published data on HBV vaccination efficacy in children under five years old in Iran from 2001 to 2014. The results showed heterogeneity in vaccine efficacy among different studies and the efficacy of hepatitis-B vaccine in under five-year-old children in different studies from Iran was 89% (95% CI: 86% - 93%). Also, the efficacy of HBV vaccination in females was somewhat more than males. Other meta-analysis studies on general populations also showed different efficacies of HBV vaccination in different provinces of Iran (8, 12, 15). This indicates the importance of launching a national study to survey HBV vaccination efficacy in different ethnic groups. According to different studies, the effect of age groups and gender on responsiveness to HBV vaccine has been controversial and still remains unclear and needs to be investigated further. The study of Tazhibi et al. showed that efficacy in adults was higher than children (15). Also Azami et al., in contrary to the results of the present study, showed that the efficacy of HBV vaccination in males was higher than females (12). Yen et al. in China did not report any relationship between age and responsiveness (27). Overall, the vaccination program was shown to be highly effective in the studied population. In other countries, similar results have been reported. For example, a study in Colombia showed 75% HBV infection reduction in children of highly endemic areas (28). In china, where the vaccination program has started from 1992, the efficacy of HBV vaccination in the general population was estimated as 88.3% (29). One reason to explain the higher responsiveness of HBV vaccination in the current study compared to others could be age of subjected population since studies showed that as vaccinated individuals grow the titer of HBV antibodies declines and older vaccinated cases need booster shots to remain protected (30). Similar to other studies, the current study had its own limitations, such as lack of access to other data resources except online data and lack of access to detailed data like hygiene conditions of subjected cases and other risk factors. Also, sources of vaccines and the antibody titration methods may be effective factors, which were not included here.
7.1. Conclusion
Generally, this meta-analysis study showed that despite a high efficacy of HBV vaccination in Iran among under five-year-old children, due to heterogenic data from different provinces, a study with national coverage is required to investigate the effective factors on vaccination efficacy.