1. Background
Primary sclerosing cholangitis (PSC) is chronic and progressive disease characterized by fibro-obliterative mechanism leading to the destruction of bile ducts and subsequently liver cirrhosis (1-3). The pathogenesis of the disease remains to be unclear and autoimmune phenomenon is suggested (1, 2). The manifestations of the disease are variable and extend from asymptomatic presentation to end-stage liver disease (1). Medical treatment failes to cure or halt the progression of PSC at this time and liver transplant remains to be the definitive treatment in severe form of PSC (2, 4, 5). IgG4-related cholangiopathy (IRC) is a recent entity and considered a manifestation of systemic inflammatory disease (1, 2, 5). Biliary strictures can develop in both diseases, although IRC appears to have distinct clinical, biochemical and histological features from PSC (6). In IRC, autoimmune pancreatitis is observed more frequently, however, PSC is associated with inflammatory bowel disease (7, 8). In addition, PSC occurs in younger patients compared to IRC which it occurs more frequently in older individuals (6, 9).
Serum plasma IgG4 level might be helpful in establishing the diagnosis of IRC (10). However, 10% of PSC patients are reported to have high serum IgG4 level (11). This small proportion of PSC patients with elevated serum plasma IgG4 level have been shown to suffer from a more severe disease course as evidenced by shorter time to liver transplantation (9). However, it is not clear whether the PSC-IgG4 phenotype resembles IRC disease prognosis. There are five cardinal diagnostic features of IRC that were subsequently applied to PSC-IgG4 phenotype: 1, histology; 2, imaging; 3, serum IgG4; 4, other organ involvement; and 5, response to steroids IRC diagnosis (6, 12).
Histological differentiation of IRC from PSC is challenging due the presence of tissue-infiltrating IgG4-postiive plasma cell deposition in both diseases (13). According to the consensus statement on the pathology of IgG4 disease, if the liver explant contains > 50 IgG-positive plasma cells/HPF and at least one histological features of dense lymphoplasmacytic infiltrate, storiform fibrosis or obliterative phlebitis is highly specific for IRC (14, 15). However, the cut-off value for IgG4 positive cells/HPF is variable across the literature (16, 17).
Liver transplantation is the only curative treatment for PSC patients with advanced liver disease (18). Several studies have shown good long-term outcomes following liver transplantation in PSC patients (18, 19). However, the outcome of liver transplantation for PSC patients is affected by recurrent disease in 20% of population (19, 20). There is a negative impact of recurrent PSC (rPSC) on Graft function and patients survival with increased risk of graft dysfunction and death among patients with rPSC (19, 21). Several risk factors for rPSC have been identified, but the impact of IgG4 on rPSC after liver transplant is still unknown (19, 22-24). The aim of our study was to determine the association between IgG4 immunochemical staining in liver explants and recurrence of primary sclerosing cholangitis post-liver transplantation.
2. Methods
2.1. Study Population
All adult patients who underwent liver transplantation for PSC at London health Sciences centre, from 1990 to 2014 were identified following the approval of the institutional board at Western University. 120 patients underwent liver transplantation secondary to PSC. However, 40 of them were excluded from the study due to the unavailability of the explanted liver for staining.
2.2. Data Collection
Clinical and demographic information were collected from the database. Data collection included recipient age, gender, presence/type of inflammatory bowel disease, type of biliary construction at the time of transplantation, incidental cholangiocarcinoma in the explant, graft failure, time to rPSC post-liver transplantation and the association between rPSC and IgG4 staining. In addition, episodes of acute cellular rejection, severity of rejection and treatment received for these rejections were reported in the study.
All patients were diagnosed with PSC prior to transplantation underwent an extensive workup with cross-sectional abdominal imaging and cholangiograms (endoscopic retrograde chaolangio-pancreatography or magnetic resonance cholangiopancreatography). Post-liver transplantation, patients are booked to have blood test in regular basis. Protocol liver biopsies are not routinely performed in our centre; however, they are obtained in liver transplant recipients with elevated liver enzymes.
Recurrent PSC was defined by the presence of characteristic features on magnetic resonance cholangiopancreatography (MRCP) or (endoscopic retrograde cholangiopancreatography) ERCP, liver biopsy in the absence of other biliary diseases, hepatic artery thrombosis, anastomotic stricture, allograft rejection or concomitant infection. MRCP features include annular biliary strictures with irregularity/beading of the biliary tree. On biopsy, PSC recurrence is defined by the presence of fibrous cholangitis and/or fibro-obliterative lesion. Graft loss was defined as death or re-transplantation, and survival was defined from the date of the LT to the date of last follow-up (censored), or date of death (uncensored).
2.3. Histological Assessment and Immunochemical Staining
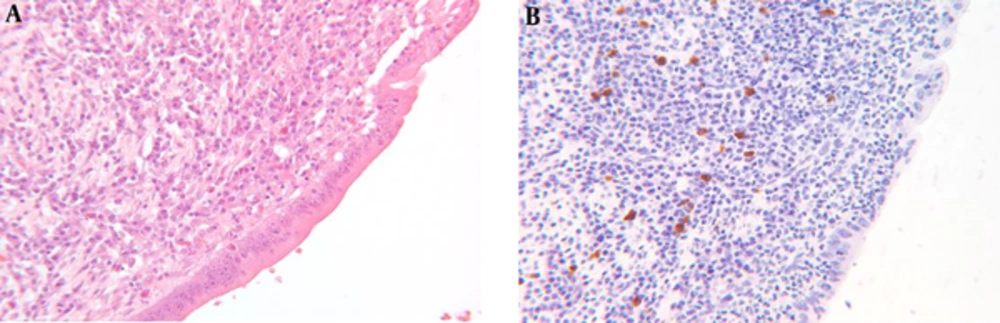
Histological evaluation was performed among PSC patients post LT. In all PSC liver explants formalin fixed paraffin embedded tissue sampling the liver hilum were retrieved. The specimens were immunohistochemically stained with anti IgG4 antibody. To quantify the IgG4 positivity within any given tissue section, the field containing the highest number of IgG4 positive cells were counted. Immunohistochemical staining is considered to be positive if the score was > 5 cells/HPF (Figure 1).
The diagnosis of recurrent PSC was made by MRCP/ERCP or the presence of liver histology compatible with primary sclerosing cholangitis, as described previously. Protocol biopsies were not performed in our center and were ordered “for cause” with elevation of liver biochemistry tests.
2.4. Statistical Analysis
Categorical variables were reported as frequencies and percentages, and continuous variables were reported as means with standard deviations (SDs) and medians. Differences between groups were examined using the t test for continuous variables and by the χ2 test (or F-test when warranted) for categorical variables. All statistical tests were two-sided and differences were considered significant when P < 0.05. Statistical analyses were performed using SAS Version 9.1.2 (SAS Inc., Cary, N.C.).
3. Results
A total of eighty patients fulfill the criteria for our study. 53 (66%) patients were male and the mean age at LT was 43.3+/- 13.4 years. 54 (76.5%) of patients had IBD and 18% of patients underwent duct-to-duct anastomosis (Table 1). PSC recurrence and graft failure occurred in 20% and 23.7%, respectively. IgG4 staining was positive in 21 subjects (> 5 cells/HPF) compared to 59 subjects who were negative for IgG4 (< 5 cells/HPF). Baseline characteristics of the IgG4 positive compared to the IgG4 negative group are presented in Table 2. Recurrent PSC was observed more frequently in the IgG4 negative group compared to the IgG4 positive group (26% vs. 5%, P < 0.009). Likewise, in time to event analysis, rPSC occurred earlier in the IgG4 negative group compared to the IgG positive group (Table 3). In addition, graft failure was higher in the IgG4 negative compared to the IgG4 positive group; however, the result was not statistically significant (21% vs. 9%, P = 0.13). Causes of graft failure are described in Table 4 and the most common cause of graft failure is rPSC.
| Variables | Value |
|---|---|
| Gender | |
| Male | 53/80 (66) |
| Female | 27/80 (34) |
| Age, mean ± SD, y | 43.3 ± 13.4 |
| Inflammatory bowel disease | 54/80 (67.5) |
| Crohn’s disease | 12/80 (15) |
| Ulcerative Colitis | 42/80 (52.5) |
| Type of anastomosis | |
| Duct-to-duct | 18(22.5) |
| Roux-en-Y | 62(77.5) |
| Recurrent PSC | 16/80 (20) |
| Graft failure | 19/80(23.5) |
| Cholangiocarcinoma | 4/80 (5) |
aValues are expressed as No. (%).
| Variables | IgG4 Positive (> 5 Cells/HPF) (N = 21) | IgG4 Negative (< 5 Cells/HPF) (N = 59) | P Value |
|---|---|---|---|
| Gender | NS | ||
| Male | 11 (50) | 42 (71) | |
| Female | 10 (50) | 17 (29) | |
| Age, mean | 43.3 | 42.2 | NS |
| Inflammatory bowel disease | 8 (38) | 18 (30) | NS |
| Crohn’s disease | 3 (14.2) | 9 (15) | |
| Ulcerative colitis | 10 (47.6) | 32 (54) | |
| Type of anastomosis | NS | ||
| Duct-to-duct | 15 (72) | 47 (80) | |
| Roux-en-Y | 6 (28) | 12 (20) | |
| Recurrent PSC | 1 (5) | 15 (26) | 0.009 |
| Graft failure | 2 (9) | 17 (21) | NS |
| Cholangiocarcinoma | 1 (4) | 3 (5) | NS |
aValues are expressed as No. (%).
| Outcome After Liver Transplantation | IgG4 Positive, (> 5 Cells/HPF), (N = 21) | IgG4 Negative, (< 5 Cells/HPF), (N = 59) |
|---|---|---|
| Recurrence during follow-up, No. (%) | 1 (5) | 15 (26) |
| Recurrence at 1-year, % | 0 | 27 |
| Recurrence at 5-years, % | 0 | 33 |
| Recurrence at 10-years, % | 5 | 60b |
aCalculated using the Kaplan-Meier method, and compared using the Log-Rank.
bSignificantly different from each other at the level of P = 0.016.
| IgG4 Positive, (> 5 Cells/HPF), (N = 21) | IgG4 Negative, (< 5 Cells/HPF), (N = 59) | |
|---|---|---|
| Recurrent PSC | 1 | 10 |
| Chronic rejection | 0 | 4 |
| Ischemic hepatitis | 1 | 1 |
| Other | 0 | 2 |
The median time for follow-up in the IgG4 positive group was 99.6 months compared to 152.6 months in the IgG4 negative group. There were 4 cases of cholangiocarcinoma in the IgG4 negative group compared to one case in the IgG4 positive group in the explanted liver (P = 0.07). However, the rejection rate was similar in both groups post-liver transplantation (25% vs. 22%, P = NS).
4. Discussion
Sclerosing cholangitis can be attributed to primary disease such as PSC or IgG4-related sclerosing cholangitis (IRC) or secondary pathology such as biliary stones or human immune deficiency cholangiopathy (11, 18). The distinction between PSC and IRC can be challenging and serum IgG4 level is not specific on differentiating between the two entities (15, 25). Therefore, a diagnostic criterion was established and it has been frequently used to diagnose IRC (3, 26, 27); despite this, histological differentiation between PSC and IRC in explanted livers can be difficult. Though the presence of > 50 IgG4 plasma cells/HPF is highly specific for IRC, but, several studies have shown good response to steroids in patients with low level of IgG4 in surgical specimens (17, 28, 29). Therefore, we used a cut-off of > 5 cell/HPF to consider the immunochemical staining is positive for IgG4 disease.
In our study, 26.3% patients had positive IgG4 staining with cut-off > 5 cells/HPF. Zen et al. similarly reported an IgG4-positive staining in 29% of PSC liver explants (13). In addition, Fischer et al. observed that 16% of PSC liver explant patients had positive IgG4 staining (24). Expectedly lower as the positive cut-off for was > 50 cells/HPF (24).
IgG4-positive staining was protective against PSC recurrence post-liver transplantation. In matter fact, recurrent PSC and graft failure were higher in IgG4 negative group compared to the IgG 4 positive group. Fischer et al., obtained IgG4 immunochemical staining in 122 PSC liver explants (24). There was no significant difference in post-operative mortality and rPSC post-liver transplant between the IgG-positive and IgG4-negative group (24). Conversely, Zhang et al. showed that rPSC was higher in the IgG4-positive group compared to the IgG4-negative group (13). The discrepancy of rPSC between the two studies might be due to the definition difference that was used on explanted specimen to define IgG4 positive patients. Tissue IgG-4 positivity was defined by the presence of > 10 IgG4 plasma cells/HPF in Zhang’s study compared to > 50 IgG4 plasma cells in the Fischer’s study (13, 24). Despite using a less restrict definition (> 5 IgG4 plasma cells/HPF) in our population to identify patients with positive IgG-4 disease, there was more PSC recurrence in the IgG-4 negative group post-liver transplantation.
In our study, the rate of cholangiocarcinoma was similar in both groups. This finding is corroborated in other study by Fischer et al. However, a significant difference in the incidence of dominant biliary strictures were observed in her study (24).
The main limitation of our study is absence of correlation between serum IgG4 level and immunochemical stain in tissue specimen. However, the utility of measuring serum IgG4 is limited by its specificity since it can be elevated in other systemic conditions. Therefore, the serum IgG4 level cannot be used in isolation to confirm the diagnosis of IRC. Boonestra et al. found that the serum IgG4 level was elevated above the limit of normal in 15% of PSC patients (30). However, the positive predictive value and the specificity increased significantly when applying the 4x upper limit of normal cut off for the IGG4 serum with a sensitivity of 42% (30). In addition, the disease can be patchy and can be missed on a regular biopsy. Therefore, histology with immunological staining in isolation cannot be used to confirm the diagnosis (30). The second limitation in our study that the histological findings of IgG4-related disease other than immunostaining are not reported in our study. Therefore, tissue positivity for IgG4 may reflect non-specific effect of chronic inflammation. Furthermore, due to the retrospective nature of our study, there may be a selection bias regarding our cohort which may represent an advanced form of recurrent PSC disease.
In conclusion, IgG4 positive staining in PSC liver explants was found to be protective against PSC recurrence, but not associated with graft survival post-liver transplantation. However, further studies are warranted to validate our result.