1. Background
Hepatitis C Virus (HCV) causes chronic infection in more than 50% of the infected individuals and may lead to fatal liver complications (1). More than 200 million people across the globe are chronically infected with HCV (2). Chronic HCV infection can lead to complex sequela, including diabetes, steatosis, cirrhosis, and ultimately hepatocellular carcinoma (HCC) (3). The primary objective of HCV treatment is to inhibit and consequently eradicate HCV RNA by having sustained virological response (SVR) against the infected virus. Treatment effectiveness is defined by the absence of detectable HCV RNA in the patient serum using the polymerase chain reaction after anti-viral treatment for six months (4). The desirability of novel broad-spectrum anti-viral drugs in the recent years has been considered as a way of providing effective treatment against a wide range of viruses, yet the risk of disease relapse has reduced R&D expenditures linked with designing and developing individual drugs targeted at individual viruses. Targeting of host factors of HCV replication is a new approach for the design and development of small inhibitors with maximum anti-HCV activity. These direct acting drugs have been shown to be effective while nucleoside/nucleotide transcriptase inhibitors are susceptible to failure due to the development of viral resistance mutations (5). Thiazolides belong to a class of inhibitors that induce phosphorylation of eIF2-alpha through PKR activation and thus block protein synthesis (6). Romark was the first to use nitazoxanide as a possible therapy against chronic HCV infection (7). Clinical trials for the therapeutic effect of this compound against chronic HCV infectivity showed that the effect of this compound involved modulation of host cellular response against the virus (8). Nitazoxanide is a thiazolide derivative, which activates the host’s protein kinase R (PKR) anti-viral pathway. Protein kinase R activation results in phosphorylation of initiation factor α (eIF2-α) of eukaryotic cells, which starts a complex network of signaling cascade that triggers an anti-HCV response in the host (9). Nitazoxanide activates PKR signaling, which results in spontaneous antiviral response. Studies on anti-HCV activity by nitazoxanide against genotype 4 have shown 80% SVR in combination with peg-interferon-alpha, versus 50% for peg-interferon-alpha alone (4, 10). In 2008, Korba et al. showed that thiazolide derivatives like nitazoxanide and tizoxanide were promising inhibitors of HBV and HCV. Currently, many studies are in progress to investigate the long-term effects of nitazoxanide, including a recent Phase 2 trial, to study the potential for the development of drug resistant mutants in HCV (11, 12).
The potential development of resistance by HCV against tizoxanide was studied in liver cell line Huh-7 transfected with genotype 1b replicons, which was later incubated with increasing amounts of tizoxanide for 24 weeks. Hepatitis C virus was not shown to develop resistance to tizoxanide and sequencing of viral genome failed to detect distinctive resistance mutations. These findings confirmed a host-mediated mechanism of action with no detectable development of viral resistance (11, 13).
Recent studies utilizing Bovine viral diarrhoea virus (BVDV) as an alternate model for HCV infectivity, have shown that nitazoxanide hinders BVDV cytosolic and non-cytosolic replication by a system that involves PKR phosphorylation of eIF2-a. It was also shown that nitazoxanide diminishes ATP-sensitive intracellular Ca2+ stores resulting in mild endoplasmic reticulum (ER) stress, which changes N-linked glycosylation of viral envelope proteins (14). Mathematical models, based on polynomial-representations of chemical compounds, can be used to predict their properties [a]. Several algebraic polynomials have useful applications [c,d, e] in chemistry, such as Hosoya polynomial (also called Wiener polynomial) (6), which play a vital role in determining distance-based topological indices. Weiner, in 1947, approximated the boiling point of alkanes as αW(G) + βP3 + γ where α, β, and γ are empirical constants, W(G) is the Weiner index, and P3 is the number of paths of length 3 in G (2, 11). Thus, Weiner laid the foundation of topological index, which is also known as the connectivity index. Many chemical experiments require determination of the chemical properties of emerging compounds, polymers, and nanomaterials. Chemical-based experiments have revealed that out of more than 145 topological indices, no single index is strong enough to determine physio-chemical properties, although, in combination, these topological indices can achieve this.
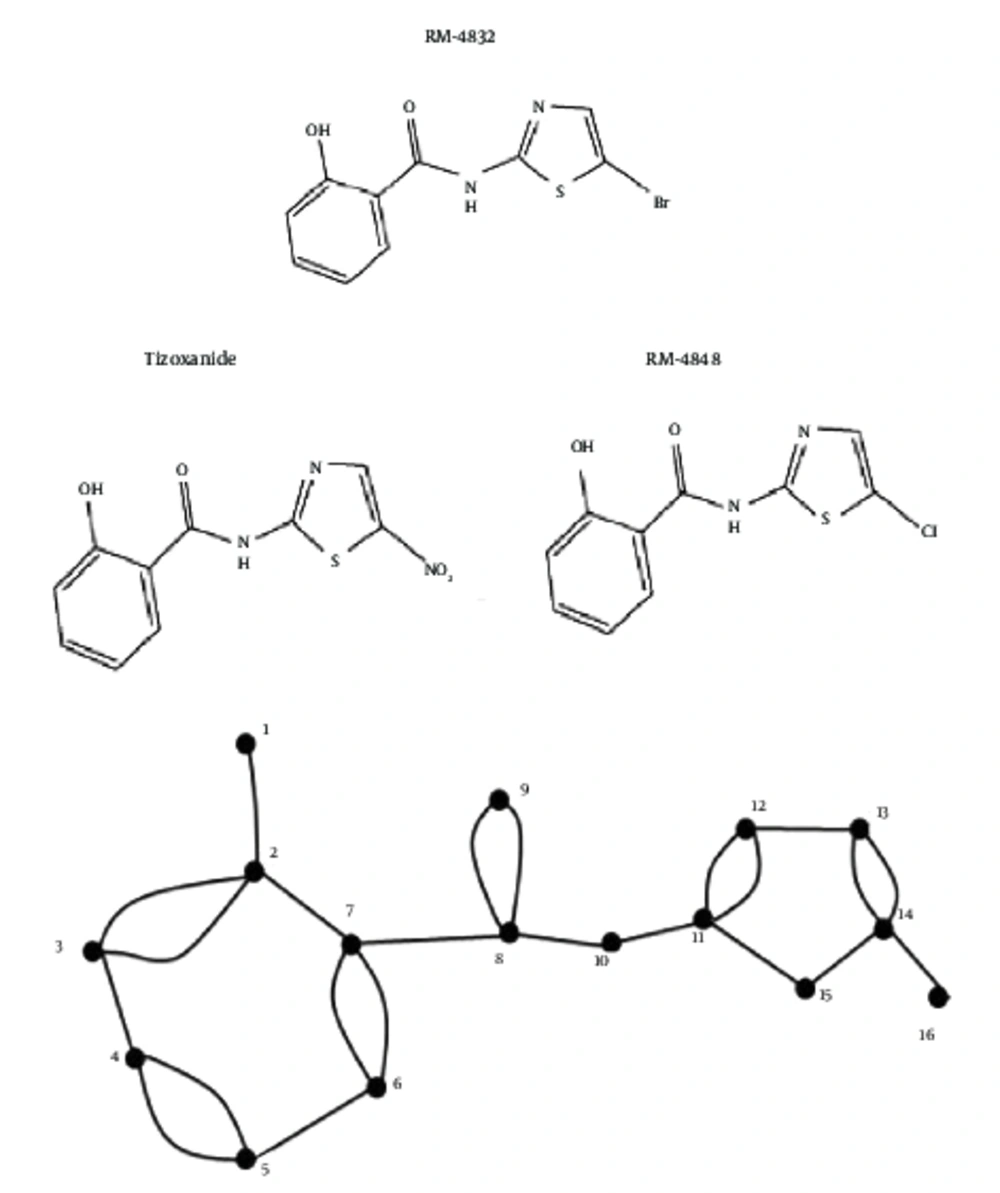
In chemical graph theory, a graph is an ordered pair of vertex set V(G) and edge set E(G). It is connected if there exists a path between any pair of vertices in G. A simple graph has no multiple edge and loops. The number of vertices of G, adjacent to a given vertex v, is the “degree” of this vertex, and will be denoted by dv(G) or, if misunderstanding is not possible, simply by dv. Distance d(u,v) is the length of the shortest path between vertices u and v. For details on basics of graph theory, any standard text, such as (12), can be of great help. Throughout this article we work on graphs with the following fixed notations:
dv is the degree of a vertex v, V{k} = {v ϵV(G)| dv = k},




And mij(G) is the number of edges of G, such that {dv, du} = {I, j}. In 1988, Hosoya conceived a graph polynomial, which he named “Wienerpolynomial”, but which most contemporary authors call the “Hosoya polynomial”. It is defined as [f], H (G,x) = Ʃk≥1 d (G, k)xk, where d (G, k) is the number of pairs of vertices of graphs k distance apart and k runs from 1 to maximum distance, also referred as the diameter of the graph.
It is a wellestablished fact that many important distance-based indices can be easily derived from Hosoya polynomial, for example [f].
W(G) = H′ (G, 1), where prime over H represents the first derivative in calculus. Similar definitions for other distance-based indices, which can be derived from Hosoya polynomial, are given in [f]. Wiener index, also called Wiener number, is a topological invariant of a molecule, defined as the sum of the lengths of the shortest path between all pairs of vertices of a graph, representing the non-hydrogen atoms in the molecule (15). The Wiener index was defined by Harry Wiener in 1947 and he called it the “path number” (16). It was the first ever topological index used for molecular branching (17). This discovery led to the calculation of many other topological indices of chemical graphs, derived from the distance matrix of the graph.
The same concept was studied in pure mathematics, mostly in graph theory, by different names like the distance of a graph, the gross status, and the transmission. The Wiener index is strongly linked to the closeness of centrality of a vertex in a graph, a quantity inversely proportional to the sum of all distances among a given vertex and all other vertices (16, 18-22).
The basic focus of the present study was to compute Hosoya polynomial and Wiener indices for 8 derivatives of thiazolides. The Wiener index could be a valuable tool for the calculation of physiochemical properties of the selected compounds, which in turn will help determine the stability of the compound in a given biological system. To the best of the author’s knowledge, this is the first study carried out to calculate the physiological properties of thiazolides, using the Wiener index.
Biologically stable compounds have the ability to stay in the body for a long time without being flushed out or degraded. In the case of interferon, by pegylated interferon, the retention of interferon inside the body increases, which decreases the dosage needed to be administered. Calculation of drug stability is a promising area of research in drug design strategies. This study can be helpful in calculating the stability of anti-viral compounds in a much more suitable and precise way prior to analyzing in vitro mechanisms of action.
2. Methods
A molecular graph was constructed with each vertex representing an atom in the structure and the search edge representing a covalent bond. Hosoya polynomial was computed for derivatives of thiazolides. First, the number of edges in the graph were computed, which actually represent d(G, 1), i.e. number of pairs of vertices one distance apart. Then, the number of pairs, which are two distance apart, wascomputed, and the same procedure was repeated for the diameter of the graph. Then by using the definition of Hosoya polynomial, its formula was obtained for a particular derivative of thiazolide. In the end, its first derivative at one, gave the Weiner index.
W (Pn) = W (Sn) = Wiener indicated that this index was closely related to the boiling points of hydrocarbons like alkanes (15). Recent studies on Weiner index showed that it can also be used to calculate physical density, surface tension, and viscosity of its liquid phase, and interactions in the molecule (20, 21).
An alternative procedure for computation of the Wiener index of a tree has been described by BojanMohar and TomazPisanski. By their method, the graphs are generalized having weights attached to the vertices, where the weight of a path is the product of its length with the weights of its two endpoints. If v is a pendent vertex of the tree, then the Wiener index of the tree is computed by adding their weights together, computing the index of the resulting smaller tree, and adding an error correction term for the paths that go through the edge from v to its parent tree. By repeatedly destroying leaves in this way, the Wiener index is calculated in real time (22).
For breaking down complex graphs into simpler graphs, the Wiener indices of such products can be calculated by a formula that unites the indices of corresponding factors (16). The Wiener index of the Benzenoids graphs, which are the Cartesian product of three trees, can be computed using the product formula with the linear time tree algorithm (17).
2.1. Ethical Committee
The ethical committee of the University of Punjab-Pakistan and University of Education Lahore also approved this study.
3. Results
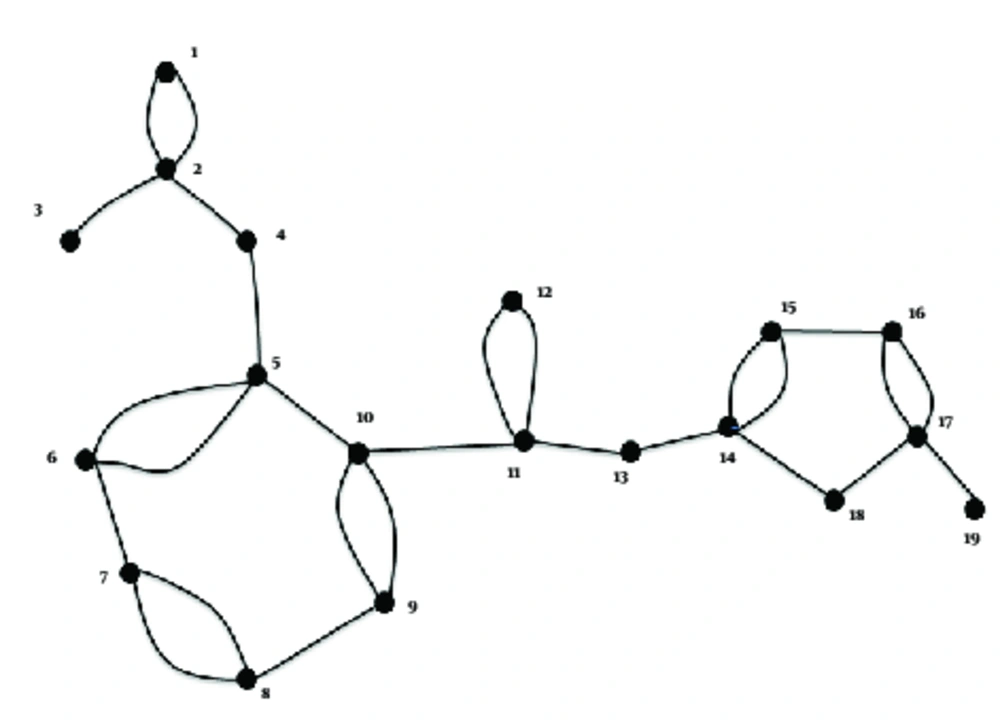
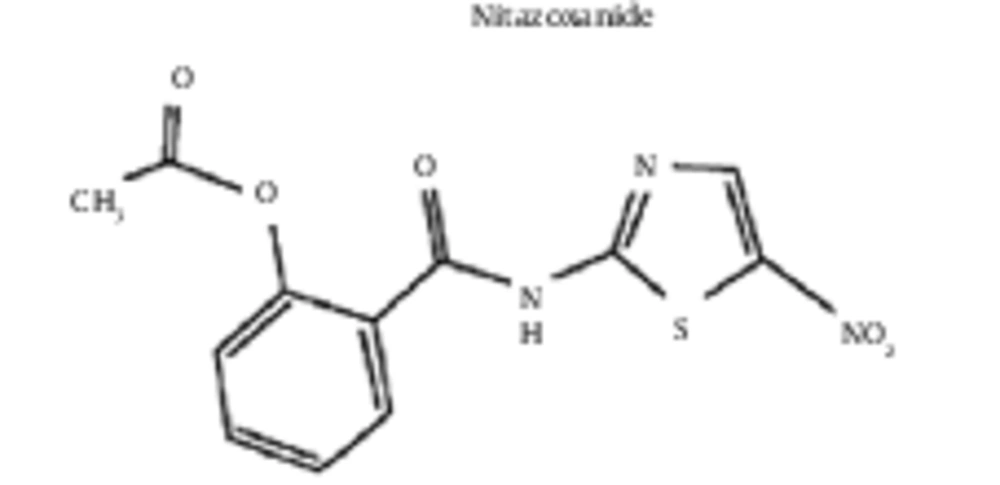
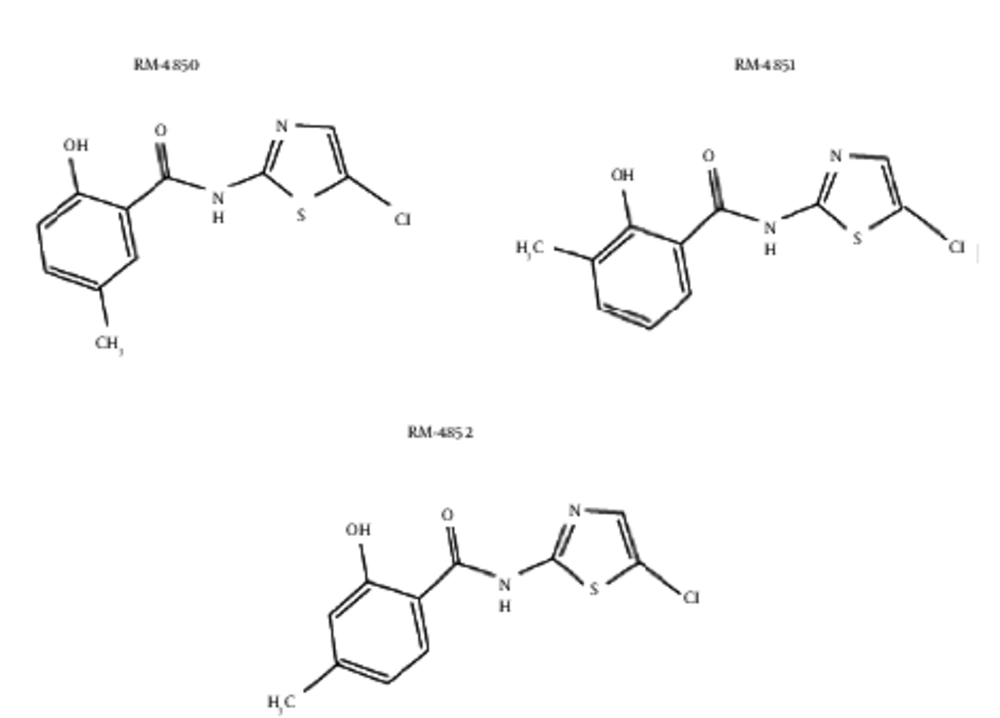
The Hosoya polynomial was computed followed by the Wiener index for the molecular graphs of eight derivatives of thiazolides i.e. RM-4832, RM-4848, RM-4850, RM-4851, RM-4852, RM-4850, tizoxanide, and nitoxanide (Figure 1), where vertices represent atoms of the structures and the edges represent the covalent bonds (Figure 2). To avoid repetition and unnecessary details, the authors only provided proof of Hosoya polynomial and Weiner index of nitaxonide and RM-4850, RM-4851, RM-4851, and left the remaining proofs to the reader. The result for Nitaxonide is given in Figure 3.
3.1. Statement 1
Hosoya polynomial and Weiner index of Nitoxanide was computed as:

and:

Proof: A: the following is the molecular graph associated with nitoxanide:
Clearly from the graph 27 edges are retrieved.
The coeffiecent of x was 27; 27 pairs were two and three distantces apart, 24 pairs were four distances, 27 pairs were three distances apart, 23 pairs were five distances apart, 20 pairs were six distance apart, 14 pairs were seven distances apart, 11 pairs were eight distances apart, 6 pairs were nine distance apart, and two pairs were 10 distances apart, which is the diameter. Thus, using the definition, we arrive at the Hosoya polynomial.
Proof: B: differentiating with respect to x and substituting x = 1, the Weiner index was obtained.
The authors computed the Hosoya Polynomial and Weiner index of RM-4850, RM-4851, and RM-4852 as their molecular graphs were the same, thus they arrived at a single result for these (Figure 4).
3.2. Statement 2
A)

B)

Proof: A: clearly the coefficient of x is 25. There are 24 pairs at two distances apart, 24 pairs at fourdistances, 27 pairs at three distances apart, 23 pairs at five distances apart, 20 pairs at six distances apart, 14 pairs at seven distances, 11 pairs at eight 8 distances, six pairs at nine distances apart, and two pairs at ten distances apart, which is the diameter. Therefore, using the definition, the Hosoya polynomial was achieved.
Proof: B: differentiating with respect to xand substituting x = 1 we obtain Weiner index.
Now we discuss next group of thiazolides which have same molecular graph (Figure 5).
3.3. Statement 3
A)

B)

Proof. proof is left to the reader.
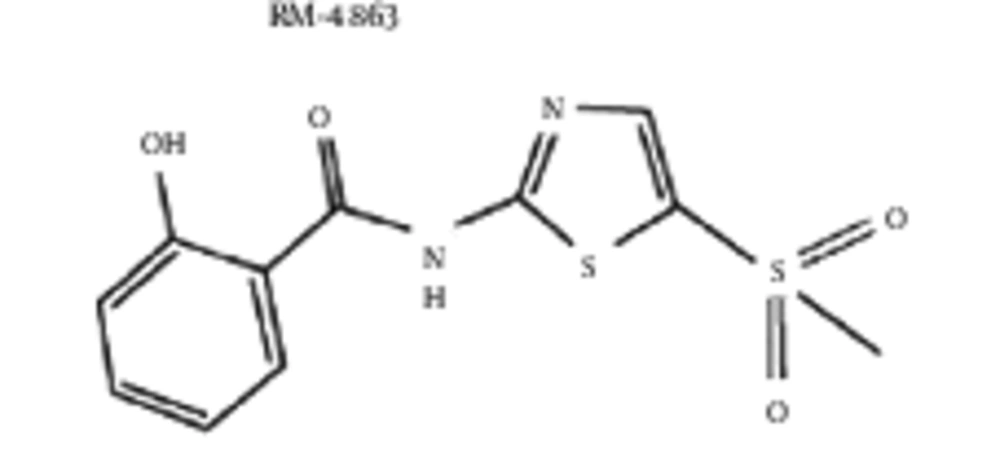
The Hosoya polynomial and Weiner index of RM-4863 is provided below (Figure 6):
3.4. Statement 4
A)

B)

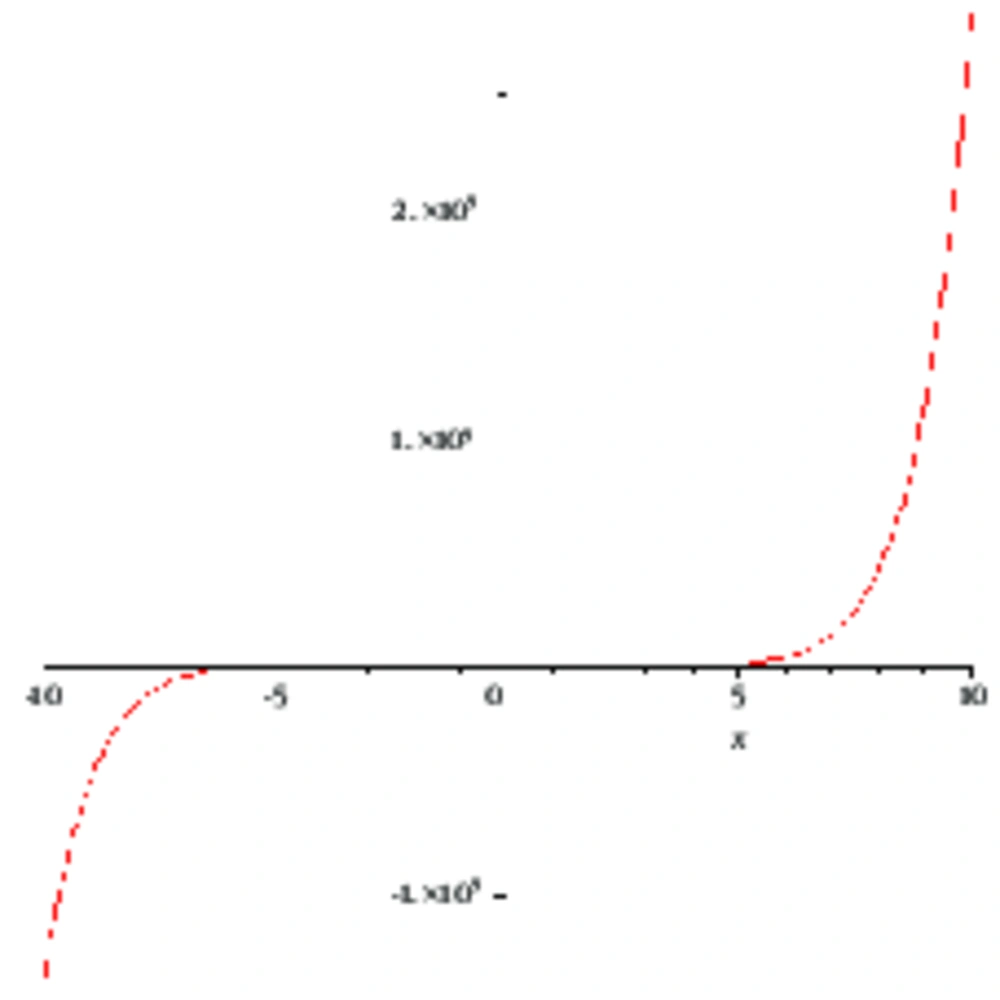
Also, it is important to mention that the computer-generated graphs of Hosoya polynomials are the same except for the slope.
4. Discussion
Romark was the first person to use these compounds for the treatment of HCV. Clinical studies on Nitazoxanide (NTZ), a prototype, for the treatment of chronic hepatitis C, have shown that all responders had low levels of serum HCV RNA (7). Thiazolide derivatives have been shown to be effective against many unicellular organisms and viruses (23, 24). Thiazolide derivatives were initially developed for the treatment of intestinal infections. These tiny drug molecules target host-mediated pathways involved in viral propagation. The anti-viral characteristics of thiazolide derivatives were discovered during research on the therapy of cryptosporidiosis in patients with human immunodeficiency virus (HIV), and further thiazolides, a novel class of anti-infective drugs, have been effective against viruses, bacteria, intracellular and extracellular protozoa, parasites, and proliferating mammalian cells (25, 26).
Later it was marketed in the USA for the treatment of diarrhoea. Nitazoxanide is safe when administered orally and has extensive post-marketing demand by millions of adults and children (27). Nitozlides interfere with Pyruvate: Ferredoxin oxidoreductase (PFOR) enzyme-dependent electron transfer reactions, which are indispensable for anaerobic energy metabolism for anaerobic bacteria (28).
Tizoxanide is another derivative of thiazolide and has been shown to be effective against Hepatitis B and C infections. Tizoxanide’s mechanism of action on HCV replication in cell culture showed that it stimulates PKR. It is a major component of non-specific immune response in host cells exposed to exogenous double-stranded RNA. This in-turn precipitates the phosphorylation of eukaryotic initiation factor 2a (eIF2-a), a gene known to hinder viral replication, by blocking protein synthesis (6, 10). Romark first showed that chronic HCV patients were cured with nitazoxanide 500 mg, administered twice daily in 50 adult individuals (7). The drug was well-tolerated with mild gastrointestinal events in the treatment and placebo groups.
Currently, nitazoxanide is used to inhibit the replication of a wide range of other RNA and DNA viruses, encompassing the encephalitis virus, respiratory syncytial virus, norovirus, coronavirus, parainfluenza rotavirus, HCV, HBV, dengue, Japanese, and HIV in vitro (25). Clinical measurements indicate a possible role for thiazolides in ongoing therapy for patients with chronic HCV and HBV. Future clinical strategies will pay attention to viral gastroenteritis, viral respiratory diseases, and dengue fever, as emerging infections (25).
Mathematical models based on molecular graphs can be used to compute the physio-chemical properties, such as radius of gyration, viscosity, heat of formation of alkanes, boiling points of hydro-carbons, fracture toughness, strainenergy, and many other properties of chemicals under investigation. These approaches have never involved utilizedon compounds, which are potent and proven antiviral compounds. The Wiener index was firstly introduced in 1947 by Harry Wiener, and Wiener called it the "path number". It is the oldest topological index linked to molecular graphing (17). Based on its achievement, numerous other topological indices of chemical graphs based on information in the distance matrix of the graph, had developed consequently to Wiener’s work.The researchers derived formulas of Hosoya polynomial for these derivatives of thiazolides. Weiner indices for these compounds were then computed. Other numerical descriptors can also be described in similar ways, see [f]. Mathematical graphs of these polynomials to draw comparison are also provided.
Weiner described the use of Weiner index to calculate the stability of molecules, like hydrocarbons, regarding boiling point and other physical properties, including surface tension viscosity and density.
These indices were used to compute the physiochemical properties of these thiazolide derivatives with a view to estimate the stability of each compound in a given biological environment. It can thus be concluded that Wiener Index strategy can be equally helpful in calculating the stability of potent anti-viral compounds.
Stability is an important prerequisite for drug design. There are a number of drugs available on the market with short half lives in a biological system. This aspect can be explained by comparing the stability of simple interferon and pegylated interferon. By pegylating the interferon, its retention time in the body increases manifold. This modification makes it more stable and available in the body for a longer time period. By making it more stable, the dosage required to initiate the threshold of antiviral response may also be decreased. The Wiener Index can help calculate the stability of derivatives of thiazolides in silico. The same principle can be used to determine the stability of other aromatic compounds. Using this approach, the time required to analyze the compounds in vitro can be decreased. In the end, it was concluded that RM-4832 has the highest Weiner index 752 so this thiazolide may play a better part in treatment of HCV and HBV, as compared to others, having low Weiner indices.