1. Background
Hepatitis C virus (HCV) is one of the leading causes of hepatitis, liver cirrhosis, and hepatocellular carcinoma (1, 2). In 2015, 1.0% of the population (71.1 million people worldwide) was estimated to be infected with HCV, indicating a significant disease burden (3). HCV is an RNA virus, classified within the Hepacivirus genus of the Flaviviridae family, with seven genotypes and 67 subtypes characterized thus far (4, 5). HCV genotype 1 is most prevalent worldwide (approximately 46%), followed by genotype 3 (approximately 30%) and genotypes 2, 4, 6, and 5 (6). Substandard health care safety measures and the use of drugs through injection are the leading causes of new HCV infections and accounted for the majority of the 1.75 million new infections in 2015 (7). A midpoint anti-HCV prevalence of 67% was observed among injecting drug users (IDUs) from 77 countries and a 47-fold higher anti-HCV prevalence compared to the general population was noted (8, 9). HCV prevalence among IDUs in the European Union countries ranges from 13.8% in Malta to 84.3% in Portugal (10). The rates in the countries around Kosovo are reported from 28.8% in Albania, 31.2% in Bosnia and Herzegovina, and 53% in Podgorica, Montenegro, to 72.1% in Skopje, Macedonia, and up to 77.4% in Serbia (11-15).
Kosovo is a small country (10,908 km2) in southeastern Europe with a population of approximately 1.8 million. Administratively, it is divided into seven regions, with 27.5% of the total population residing in the largest region of Prishtina (16). Currently, data on the HCV prevalence and HCV genotype distribution among the general population, as well as specific risk groups (such as IDUs), in Kosovo are scarce. It was estimated that 4,973 IDUs were present in Kosovo following programmatic mapping and size estimation of key populations conducted in 2016 as part of the HIV program in Kosovo. Of these, 1,217 (24.5%) were from the municipality of Prishtina (17). The aim of our study was to determine the rates of HCV infection among IDUs in Prishtina, Kosovo and for the first time, to investigate the distribution of HCV genotypes/subtypes. In addition, we wished to characterize the factors contributing to HCV transmission and observe epidemiologic links using phylogenetic analyses. These data will aid in the development of appropriate HCV prevention strategies and treatment guidelines for IDUs in Prishtina, Kosovo.
2. Methods
Epidemiological data and blood samples from 205 IDUs obtained during the Biological and Behavioral Surveillance Study (BioBSS) in the Prishtina region of Kosovo were included in this investigation. The BioBSS was implemented during 2011 under the auspices of the Ministry of Health and funded by the Global Fund for AIDS, TB, and Malaria (GFATM) using respondent-driven sampling (RDS). The study inclusion criteria were: (a) age between 18 and 50 years, (b) fluency in Albanian, (c) injected drugs at least once in the past month, and (d) residing and/or working in Prishtina (or having injected drugs regularly for at least three months in the past year in Prishtina). Blood samples from IDUs were collected by the staff of the non-governmental organization Labyrinth at their premises in Prishtina. This organization provides methadone substitution therapy and needle/syringe exchange to IDUs in the Prishtina region. All eligible individuals were informed about the nature and requirements of the study and were asked for informed consent. The samples were tested using the HCV Ab Sensitive ELISA test (Dialab, Wiener Neudorf, Austria) for the detection of antibodies against HCV following the manufacturer’s instructions as part of the initial BioBSS. Serum samples that tested anti-HCV positive were included in this study to further explore HCV molecular epidemiology among IDUs in Kosovo. Data obtained from questionnaires were reanalyzed, this time mainly focusing on HCV status; that is, drug-use practices, sexual behavior, imprisonment, and opioid substitution therapy information were reviewed. This study was approved by the Ethics Committee of the Medical Faculty at the Hasan Prishtina University of Prishtina.
2.1. Molecular Testing
The presence of HCV RNA was first analyzed using the automated COBAS AmpliPrep/COBAS AMPLICOR HCV test, version 2.0 (Roche Diagnostics, Basel, Switzerland). If determined to be HCV RNA positive, viral RNA was extracted from 200 to 400 µl of plasma using the MagnaPure Compact Nucleic Acid Isolation kit (Roche Diagnostics) on the MagNA Pure Compact instrument (Roche Diagnostics). The HCV genotype was determined by sequencing the core region of the HCV genome. Briefly, PCR was performed using the SuperScriptTM III One-Step RT-PCR System with Platinum® Taq DNA Polymerase High Fidelity (Invitrogen, Carlsbad, CA, USA) and primers Sc2/Ac2, as described previously (18). If no amplicon was obtained, nested PCR with S7/A5 primers (18) and the FastStart High Fidelity PCR System (Roche Diagnostics) were employed. The 441 bp or 355 bp amplicons obtained were purified with the addition of the enzymes Exonuclease I and Shrimp Alkaline Phosphatase (Thermo Fisher Scientific, Waltham, MA, USA) and sequenced using the BigDye® Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA) by using the S7 primer. The DyeEx 2.0 Spin kit (Qiagen, Hilden, Germany) was used for the removal of unincorporated dye terminators from the sequencing reactions. Sequencing was performed on the 3500 Genetic Analyzer (Applied Biosystems). HCV genotype was determined using the NCBI Genotyping tool (19), with the addition of the genotype reference set 2012 of the core region, retrieved from HCV Sequence Alignments (20).
2.2. Phylogenetic Analysis
To investigate the molecular epidemiology and transmission routes of HCV, the NS5B region of the viral genome was sequenced as described above. Samples genotyped as subtypes 1a and 3a were further analyzed with primers A1b/F1b for subtype 1a and primers A3a/F3a and B3a/E3a for subtype 3a (21). Separate alignments of the obtained sequences were made using ClustalW, which is available in the BioEdit package (22). A quick neighbor-joining tree was created using Mega 5.05 with 100 bootstrap replicates (23). Major clusters were identified and the most similar sequences to the clustered sequences were selected by employing the HCV BLAST search tool (24-26). Genotype reference set 2014 was retrieved from HCV Sequence Alignments (20). Finally, maximum likelihood phylogenetic trees were constructed using PhyML 3.0 (27) with integrated model selection (Smart Model Selection) according to the Akaike Information Criterion. The phylogeny obtained was viewed using FigTree, v1.4.3, and phylogenetic clusters were identified according to the approximate likelihood ratio test (aLRT) branch support values obtained.
2.3. Statistical Analysis
The statistical analyses were performed using SPSS software, version 17.0. Data obtained from the questionnaires were analyzed with the focus on factors that could have contributed to HCV infection. Data were expressed as the mean ± standard deviation (SD), median, and inter-quartile range, or frequencies, as appropriate. Differences between subgroups were assessed by χ2 (Chi-square) test, Fisher’s exact test, Student’s t-test, and an analysis of variance test. Different variables were analyzed using Poisson’s multivariate regression analysis with a robust 95% confidence interval, which directly estimates the relative risk (RR). The Wald Chi-square test was used as a test for the model effect to determine the statistical significance of each of the independent variables. A P value of less than 0.05 was considered statistically significant.
3. Results
Among the 205 IDUs included in the study, the majority was men (89.3%), with a mean age of 36.2 years. The age group of 30 - 39 years was most represented (50.7%) (Table 1). During the three months prior to the interview, the majority of the IDUs lived in their own houses/apartments (42.5%) or lived with their parents (32.5%). Four subjects that responded were in prison at that time. The largest proportion of IDUs (39%) was financially supported by their family, approximately one-third (29.8%) was permanently employed, and approximately 9% had no income during the month prior to the interview. Of the total 205 IDUs participating in the study, 99 (48.3%) were anti-HCV positive. Anti-HCV positivity was significantly associated with age, marital status, and level of education (P = 0.001, P = 0.048, and P = 0.001 - 0.030). A significant association was observed between the duration and frequency of injection drug use and anti-HCV positivity (Table 1). All 10 addicts that reported drug injection four or more times per day were anti-HCV positive. The lowest HCV prevalence was noted among IDUs that injected drugs only once, or two to three times per month (31.3% and 27.8%, respectively). IDUs that injected drugs at “shooting galleries” or similar social settings were more likely to be anti-HCV positive and had a three-fold greater risk of anti-HCV positivity compared to IDUs injecting at home (Table 1).
| Explanatory Variables | n | % | Anti-HCV Positive (%) | Unadjusted OR | 95% CI | P Valuea | Adjusted OR | 95% CI | P Valuea |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Female | 21 | 10.2 | 33.3 | (1) | (1) | ||||
| Male | 183 | 89.3 | 49.7 | 1.978 | 0.763 - 5.128 | 0.160 | 1.770 | 0.653 - 4.800 | 0.260 |
| Missing | 1 | 0.5 | |||||||
| Age, years | 1.111 | 1.062 - 1.163 | 0.001** | 1.086 | 1.021 - 1.156 | 0.009** | |||
| Age, years | |||||||||
| 19 - 29 | 37 | 18.0 | 24.3 | (1) | (1) | ||||
| 30 - 39 | 104 | 50.7 | 46.2 | 2.667 | 1.146 - 6.203 | 0.023* | 2.297 | 0.873 - 5.331 | 0.096 |
| ≥ 40 | 64 | 31.2 | 65.6 | 5.939 | 2.388 - 14.772 | 0.001** | 3.221 | 0.949 - 5.563 | 0.065 |
| Marital status | |||||||||
| Not married | 142 | 69.3 | 43.7 | (1) | (1) | ||||
| Married | 63 | 30.7 | 58.7 | 1.836 | 1.006 - 3.350 | 0.048* | 1.173 | 0.472 - 2.914 | 0.730 |
| Education level | |||||||||
| ≥ Higher | 35 | 17.1 | 25.7 | (1) | (1) | ||||
| Secondary | 120 | 58.5 | 46.7 | 2.528 | 1.093 - 5.747 | 0.030* | 1.710 | 0.682 - 4.289 | 0.253 |
| ≤ Primary | 50 | 24.4 | 68 | 6.139 | 2.343 - 16.083 | 0.001** | 2.886 | 1.199 - 8.330 | 0.039* |
| Employment | |||||||||
| Employed | 61 | 29.8 | 41 | (1) | (1) | ||||
| Unemployed | 144 | 70.2 | 51.4 | 1.522 | 0.831 - 2.790 | 0.174 | 1.324 | 0.668 - 2.621 | 0.420 |
| Duration of drug use, years | 1.384 | 1.245 - 1.538 | 0.001** | 1.072 | 1.025 - 1.121 | 0.002** | |||
| Duration of drug use, years | |||||||||
| 5 - 9 | 69 | 33.7 | 33.3 | (1) | (1) | ||||
| 10 - 14 | 52 | 25.4 | 48.1 | 1.852 | 0.884 - 3.878 | 0.102 | 1.777 | 0.810 - 3.898 | 0.151 |
| 15 - 19 | 45 | 22.0 | 60 | 3.000 | 1.377 - 6.535 | 0.006** | 2.119 | 0.924 - 4.863 | 0.076 |
| 20 - 24 | 28 | 13.7 | 60.7 | 3.091 | 1.246 - 7.669 | 0.015* | 1.342 | 0.487 - 3.699 | 0.569 |
| 25 - 29 | 4 | 2.0 | 75 | 6.000 | 0.591 - 20.924 | 0.130 | 1.746 | 0.157 - 19.417 | 0.650 |
| > 30 | 7 | 3.4 | 57.1 | 2.667 | 0.55 - 12.926 | 0.223 | 0.362 | 0.056 - 2.344 | 0.286 |
| Frequency of injection | |||||||||
| Once per month | 67 | 32.7 | 31.3 | (1) | (1) | ||||
| 2 - 3 times per month | 36 | 17.6 | 27.8 | 0.842 | 0.347 - 2.059 | 0.707 | 0.991 | 0.393 - 2.499 | 0.985 |
| 1 - 3 times a week | 43 | 21.0 | 55.8 | 2.267 | 1.252 - 6.114 | 0.012* | 1.370 | 0.942 - 5.393 | 0.620 |
| 4 - 6 times a week | 6 | 2.9 | 83.3 | 10.952 | 1.204 - 9.660 | 0.034* | 1.070 | 0.411 - 2.788 | 0.890 |
| 1 - 3 times a day | 43 | 21.0 | 67.4 | 4.537 | 1.997 - 10.308 | 0.001** | 1.676 | 0.997 - 9.660 | 0.820 |
| 4 or more times a day | 10 | 4.9 | 100 | 4.00E + 09 | 0 | 0.999 | 3.00E + 09 | 0 | *** |
| Place of injecting drugs | |||||||||
| At home | 161 | 78.5 | 47.8 | (1) | (1) | ||||
| On the street or in the park | 17 | 8.3 | 41.2 | 0.778 | 0.281 - 2.150 | 0.628 | 0.764 | 0.273 - 2.142 | 0.609 |
| In a shooting gallery or other places where IDUs gather | 58 | 28.3 | 67.2 | 2.882 | 1.499 - 5.540 | 0.002** | 3.011 | 1.542 - 5.878 | 0.001** |
| Other places | 18 | 8.8 | 50 | 1.091 | 0.560 - 2.125 | 0.798 | 1.098 | 0.552 - 2.186 | 0.820 |
| Drugs | |||||||||
| Heroin | |||||||||
| No | 4 | 2.0 | 25 | (1) | (1) | ||||
| Yes | 201 | 98.0 | 48.8 | 2.854 | 0.292 - 27.906 | 0.367 | 1.711 | 0.171 - 17.158 | 0.648 |
| Cocaine | |||||||||
| No | 150 | 73.2 | 45.3 | (1) | (1) | 0.728 - 2.719 | |||
| Yes | 55 | 26.8 | 54.5 | 1.409 | 0.758 - 2.620 | 0.279 | 1.407 | 0.310 | |
| Heroin and cocaine at the same time | |||||||||
| No | 153 | 74.6 | 43.1 | (1) | (1) | 0.920 - 3.619 | |||
| Yes | 52 | 25.4 | 63.5 | 2.289 | 1.197 - 4.380 | 0.012* | 1.825 | 0.085 | |
| Morphine | |||||||||
| No | 184 | 89.8 | 44.6 | (1) | 1.369 - 11.076 | (1) | 0.802 - 8.456 | ||
| Yes | 21 | 10.2 | 76.7 | 3.894 | 0.011* | 2.604 | 0.110 | ||
| Opium | |||||||||
| No | 194 | 94.6 | 47.4 | (1) | 0.777 - 11.719 | (1) | 0.318 - 6.405 | ||
| Yes | 11 | 5.4 | 72.7 | 3.018 | 0.110 | 1.428 | 0.640 | ||
| Methadone | |||||||||
| No | 62 | 30.2 | 16.1 | (1) | 4.022 - 18.262 | (1) | 0.911 - 10.560 | ||
| Yes | 143 | 69.8 | 62.2 | 8.57 | 0.001** | 3.720 | 0.140 | ||
| Use of secondhand needles/syringes | |||||||||
| No | 181 | 88.3 | 45.9 | (1) | (1) | ||||
| Yes | 24 | 11.7 | 66.7 | 2.361 | 0.962 - 5.795 | 0.061 | 2.307 | 0.905 - 5.881 | 0.080 |
| Arrested for drug use | |||||||||
| No | 102 | 49.8 | 40.2 | (1) | (1) | ||||
| Yes | 103 | 50.2 | 56.3 | 1.918 | 1.101 - 3.341 | 0.022* | 1.679 | 0.917 - 3.075 | 0.093 |
| Past imprisonment | |||||||||
| No | 95 | 46.3 | 33.7 | (1) | (1) | ||||
| Yes | 110 | 53.7 | 60.9 | 3.068 | 1.713 - 5.438 | 0.001** | 2.260 | 1.215 - 4.204 | 0.010* |
| Sexual intercourse in the last three months | |||||||||
| No | 54 | 26.3 | 44.4 | (1) | (1) | ||||
| Yes | 149 | 72.7 | 50.3 | 1.09 | 0.582 - 2.043 | 0.788 | 1.117 | 0.966 - 1.171 | 0.737 |
| Missing | 2 | 1.0 | |||||||
| Sex for money | |||||||||
| No | 190 | 92.7 | 48.2 | (1) | (1) | ||||
| Yes | 11 | 5.4 | 63.6 | 1.265 | 0.373 - 4.288 | 0.706 | 1.033 | 0.294 - 3.625 | 0.959 |
| Missing | 4 | 1.9 | |||||||
| Opioid substitution therapy | |||||||||
| No | 100 | 48.8 | 38.9 | (1) | (1) | ||||
| Yes | 104 | 50.7 | 57.7 | 1.272 | 0.732 - 2.211 | 0.394 | 1.132 | 0.639 - 2.006 | 0.672 |
| Missing | 1 | 0.5 |
Univariate and Multivariate Logistic Regression Model to Predict HCV Infection among IDUs in Prishtina, Kosovo
A statistically significant association was observed between anti-HCV positivity and the concomitant use of heroin and cocaine, morphine, and methadone (Table 1). There was no significant difference between anti-HCV positive and negative individuals regarding injection with the use of secondhand needles and syringes, use of sterile needles/syringes, the frequency of using sterile needles/syringes, and the method by which needles were cleaned. The anti-HCV positive drug addicts more regularly obtained sterile syringes/needles from nongovernmental organizations than the anti-HCV negative IDUs (58.8% vs. 41.2%; P < 0.001). Over half of the IDUs included had been arrested for drug use or had been imprisoned in the past, and a significantly higher number of those were anti-HCV positive (Table 1). However, there was no significant association between anti-HCV positivity and injection of drugs while in prison (data not shown).
Multivariate logistic regression analysis indicated the following factors were significantly associated with HCV infection among IDUs: (1) older age (P = 0.009), (2) low education level (P = 0.039), (3) longer duration of drug use (P = 0.002), (4) drug injection at shooting galleries (P = 0.001), and (5) imprisonment (P = 0.010).
HCV RNA was detected in 70/99 (70.7%) anti-HCV positive IDUs from Prishtina. HCV genotyping was successfully performed for all HCV RNA positive IDUs, and the following subtypes were identified: subtype 1a in 45 patients (64.3%), subtype 3a in 24 patients (34.3%), and subtype 2k in one patient (1.4%).
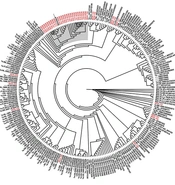
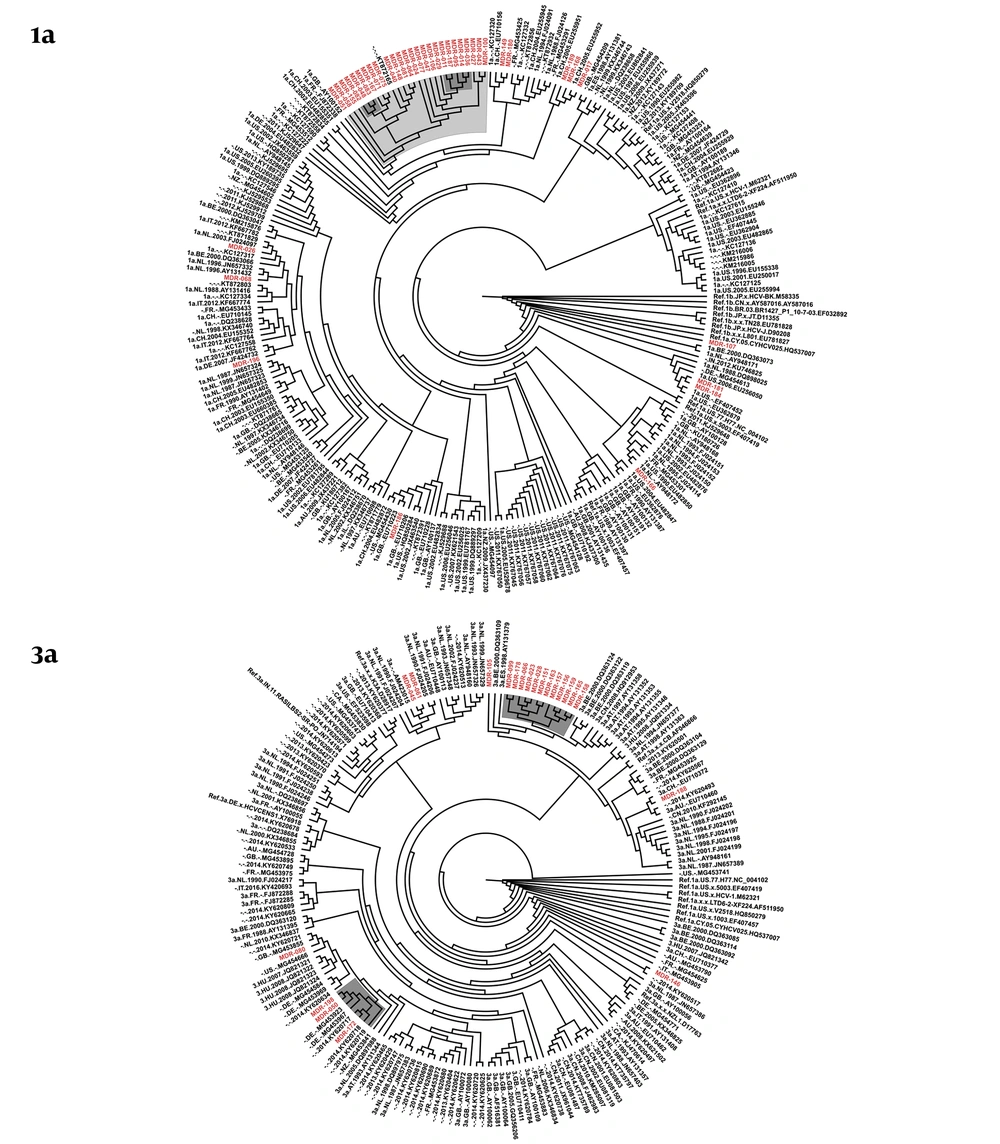
NS5B sequences were successfully obtained for 39/45 of IDUs with HCV subtype 1a and for 21/24 IDUs with HCV subtype 3a. The phylogenetic tree of HCV subtype 1a from Prishtina IDUs displayed two phylogenetic clusters with aLRT > 0.9, one with five sequences, and another with three sequences. These two clusters were part of a larger cluster with aLRT > 0.8, encompassing sequences obtained from a total of 26 IDUs. Thus, 66.7% (26/39) of the HCV subtype 1a IDUs’ samples were observed with a phylogenetic link. In addition, two transmission pairs of IDUs were seen on this cladogram (Figure 1).
The phylogenetic tree of HCV subtype 3a showed one large cluster with aLRT > 0.9, encompassing 12 sequences, another cluster with aLRT > 0.9 with three sequences from Kosovo, and one transmission pair. In total, 71.4% (15/21) of the HCV subtype 3a samples obtained from Prishtina IDUs showed phylogenetic clustering (Figure 1).
4. Discussion
Nearly half of the IDUs in the Prishtina region of Kosovo had antibodies against HCV, mainly due to active HCV infection. HCV subtype 1a was found to be most prevalent, followed by subtype 3a. In addition, this study found a high proportion of sampled individuals with a phylogenetic link to one another.
HCV prevalence has historically been high among IDUs, as observed in Kosovo and the surrounding countries (11-15). The problem of HCV infection among IDUs is pronounced in eastern and southeastern Europe, where the rate of injecting drug use is almost five times higher than the global average (28).
Subtypes 1a and 3a have been determined to be the most prevalent subtypes among IDUs in several studies (29, 30), as seen in this study. High phylogenetic clustering of the subtype 1a and 3a sequences was observed in this investigation, indicating HCV transmission within the community of IDUs residing and injecting drugs in Prishtina. It should be noted, however, that the participants in this study were selected by respondent-driven sampling (RDS), which could have contributed to the observed phylogenetic clustering.
Several factors were identified to be significantly associated with HCV infection among IDUs from Prishtina in this study; namely, older age, low education, imprisonment, and injecting drugs at shooting galleries. These factors have already been identified in other similar studies to be related to HCV transmission among IDUs (31, 32). Older IDUs were more often diagnosed with HCV infection, probably resulting from a longer duration of drug use, determined as another significant predictor for HCV infection. In addition, individuals with poor education have limited opportunities for employment in Kosovo, where the unemployment rate is high even in the general population. The issue of imprisonment and HCV infection in Kosovo requires additional investigation. The extent of drug use and the prevalence of HCV infection in prisons, as well as whether the imprisonment of IDUs is mainly due to drug-related charges, should be determined.
An important finding from this study is the relevance of shooting galleries on HCV infection among IDUs in Prishtina, as seen in other previously published studies (33, 34). This type of venue is associated with an increased risk of HCV acquisition, either via socializing with an extended group of IDUs or via the intentional or accidental use of secondhand injecting equipment.
Sharing drug-injecting equipment is linked with the social and economic situation of the user and it contributes to HCV transmission among IDUs (35). In addition to infection via contaminated needles, HCV transmission can also occur through contact with the infected liquid in syringes and inanimate surfaces, as under certain conditions, the virus can remain infective for weeks (36, 37). The results from this study show that almost all IDUs report that they use sterile needles/syringes for drug injection, but at the same time, the majority of IDUs report that somebody else also used their needles/syringes. This could imply that the study inadvertently omitted the majority of IDUs that are injecting drugs with used needles/syringes or it could indicate misreporting of sharing needles/syringes. The latter is more likely because most study participants used harm-reduction services from the Labyrinth NGO. The same staff instructed IDUs not to share used needles/syringes and conducted the interviews reported in this study. Almost all IDUs reported that they purchased sterile needles/syringes from pharmacies, whereas anti-HCV positive IDUs received sterile needles significantly more often from NGOs compared to anti-HCV negative IDUs. Considering the high unemployment rate among IDUs, purchasing sterile needles/syringes is challenging. Thus, a needle/syringe exchange program implemented through organizations working on harm reduction is of utmost importance and, together with opioid substitution treatment (OST), is the key to the prevention and control of infectious diseases among IDUs. Unfortunately, the harm-reduction program for IDUs in Kosovo is limited in its experience and scope of coverage (38). Therefore, additional resources and staff should be allocated to the already existing harm-reduction programs in Prishtina to enhance their scope of coverage and to improve their quality. Shooting galleries were shown to contribute significantly to the spread of HCV infection and should thus be prioritized for intervention. An emphasis should be placed on preventing HCV infection among younger IDUs because they have a lower rate of HCV infection compared to older groups, presenting a window of opportunity. In addition, treatment, as well as prevention, should be directed at IDUs with an active HCV infection to limit further spread of HCV in Kosovo.