1. Context
Chronic hepatitis B virus (HBV) infection has posed a serious threat to the public health in recent years. Based on statistics, there were approximately 257 million people living with chronic HBV infection worldwide in 2015 with about 86 million HBV carriers in China and also 20 million patients with chronic hepatitis B (CHB). HBV is resulted in an estimated 887,000 deaths, mostly from cirrhosis and hepatocellular carcinoma (primary liver cancer) every year (1).
Mother-to-child transmission (MTCT) is one of the main modes of HBV transmission (2). It has reported that 50% of the patients infected with HBV are resulted from MTCT (3). The risk infection with chronic HBV after acute exposure ranges from 90% in newborns of hepatitis B envelop antigen (HBeAg)-positive mothers to 25% - 30% in infants and children younger than 5 years to less than 5% in adults (4). Without immunization, 30% - 42% of the infants had chronic HBV infection who were born to hepatitis B surface antigen (HBsAg)-positive mothers and 70% - 90% of the infants became HBV carriers who were born to HBsAg, HBeAg positive mothers (5, 6).
This study aimed at reviewing the mechanisms of MTCT and controversial issues in antiviral therapy for pregnant women with high viral load in order to provide clinicians with some strategies for preventing MTCT of HBV.
2. Evidence Acquisition
Relevant published papers in English were searched using PubMed and EMBASE databases from January 2000 to January 2019. We used the following keywords in our search: mechanism, hepatitis B virus, HBV, mother-to-child transmission, mother-to-infant transmission, vertical transmission, pregnancy, tenofovir, lamivudine (LAM), telbivudine (LDT) and so on. Finally, 61 papers were included based on the controversial issues of the studies.
3. Results
3.1. The Mechanism of MTCT of HBV
Theoretically, there are three possible routes for MTCT transmission: prenatal transmission, natal transmission and postnatal transmission (7). As the pregnant women and infants are provided with passive-active immunization, the natal and postnatal transmission have significantly controlled (8).
3.1.1. Prenatal Transmission (Intrapartum Infection)
Although the exact mechanism of prenatal infection remains unclear, several hypotheses have been reported:
3.1.1.1. A Breach in the Placental Barrier
The destruction of the placental barrier is caused by uterine contractions in the process of premature delivery or spontaneous abortion, resulting in the lack of blood of HBeAg-positive mothers through the placenta. The placenta infected by HBV may undergo pathological changes, such as villi fibrinoid necrosis and hyperplasia of villi capillary hyperemia expansion leading to the weakening the placenta immune function. As a result, the fetus is infected with HBV through the placenta leakage pathway. In amniocentesis, the needle penetrates the abdominal and uterus wall, bringing blood into the uterine cavity. It has not yet determined whether invasive procedures during pregnancy can increase the risk of HBV infection in the infants (9, 10).
Two studies, including 21 and 47 HBsAg mother-infant pairs, respectively demonstrated amniocentesis as the low risk of HBV transmission (11). However, Yi et al. enrolled 642 consecutive infants, including 63 with amniocentesis and the remaining without amniocentesis. The vertical transmission (VT) rate in infants with amniocentesis was higher than those without amniocentesis (6.35% vs. 2.53%, respectively; P = 0.226). Nevertheless, the VT rate in the amniocentesis group was significantly higher than the control group, when the maternal HBV DNA levels were ≥ 7log10 copies/mL (50% vs. 4.5%, respectively, P = 0.006) (11, 12). Therefore, amniocentesis in HBsAg-positive pregnant women needs to assess the VT rate based on HBV DNA levels.
3.1.1.2. The Theory of Placental Infection
The placenta infection is considered by some researchers as the mechanism of HBV intrauterine infection (13, 14). Using immunohistochemical method to detect each layer of cells of the infected placenta, the decreased rate of HBV infection from the maternal side of the placenta to the fetal side (trend test P = 0.0009) as well as a significant correlation between intrapartum HBV infection and villi capillary endothelial cells (VCEC) (OR = 18.46, P = 0.0002) were found. It was also speculated that HBV may be infected through “cell transfer”. In other words, the mother blood directly infected villous trophoblast cells by decidual capillary endothelial and decidual cells and/or fluffy clearance. Then, villi mesenchymal cells and VCEC were further infected leading to fetal intrapartum infection (15).
3.1.1.3. Peripheral Blood Mononuclear Cell (PBMC) Infection Theory
Recent studies have shown that HBV DNA and covalent closed circular DNA (cccDNA) can be detected in peripheral blood mononuclear cell (PBMC) of the HBsAg-positive mothers. This confirms PBMC to be an important site for HBV to replicate in the extrahepatic tissue (16). PBMC is able to move freely within the tissue clearance because of its deformation and migration characteristics. In normal or pathological pregnancy, fetal infection may be caused by a small amount of maternal PBMC through the placental barrier.
3.1.1.4. The Fertilized Egg Infected with HBV
Studies have proved the existence of HBsAg, HBcAg and HBV DNA in sperm of male or oocytes of female subjects. Chen et al. detected HBV DNA in stillbirth embryonic tissues at 46 days of gestation, whereas the corresponding endometrial tissue was HBV DNA negative. The placenta had not been formed yet and embryonic blood circulation was still in the bud. Therefore, there was little possibility of HBV transmission via placenta. It was believed that HBV may be derived from fertilized egg cells.
3.1.1.5. Vaginal Ascending Infection
Vagina has been considered by some researchers as one of the channels to infect the fetus upstream HBV. Based on the detection of 59 placenta tissues of HBsAg-positive mothers, Yue et al. found that the numbers of HBsAg-positive and HBeAg-positive cells in the placental tissues of 4 pregnant women decreased from villous capillary endothelial cell side to decidua cell side. Particularly, a gradual decrease in the stain strength of the positive cells was observed. Moreover, HBsAg and HBeAg in the fetal amniotic epithelial cells were detected, in which positive HBV DNA in the vaginal secretion of the amniotic fluid was found. It confirmed that HBV could infect the fetus via vaginal ascending and also HBV in vaginal secretions could successfully infect the fetal membranes, amniotic fluid and fetus and finally each layer in the placental tissue cells (17).
3.1.2. Natal Transmission
The mechanisms underlying natal transmission include swallowing amniotic fluid, vaginal secretions or exposure to maternal blood during vaginal delivery (18). However, no evidence convinces that the natal transmission originates from ingested vaginal secretion at the time of birth. HBsAg can be detected in the gastric lavage of 90% infants born to HBV infected mothers, probably due to the integrity of the oral and gastric mucosa (19, 20).
Researchers have confirmed that the natal transmission is closely related to the length of the first stage of labor (especially lasts for 9 hours). During childbirth, due to the mixing of fetal and maternal blood (microtransfusion), leakage and damage in parts of placenta caused by the instrument increases the rate of MTCT (21-23).
Some scholars regard vaginal delivery as the risk of HBV infection in the infants. Cesarean section is suggested to use in pregnant women with high HBV DNA levels (23, 24). In 2018, a meta-analysis conducted by Yang et al. proved that cesarean section was able to reduce the risk of MTCT of HBV in comparison to vaginal delivery in China. This analysis included 28 articles and contained 30 datasets. The overall MTCT rate of HBV was 6.76% (670/906), of which the MTCT rate of cesarean section was 4.37% (223/5105), and the MTCT rate of vaginal delivery was 9.31% (447/4801). This indicates that cesarean section can significantly reduce the MTCT of HBV compared to the vaginal delivery (25).
3.1.3. Postnatal Transmission
Postpartum infection refers to the situation that infants are infected through milk, saliva or other close contact with HBV-positive mothers with HBV infection. Breast-feeding in pregnant women needs further discussion. HBsAg was detected in 72% of breast milk samples and transmitted especially when mothers had abrasion on nipple (26). If there is an increase in intestinal mucosal injury or permeability in infants, HBV of breast milk may also enter the blood circulation through capillary network, causing neonatal or infant HBV infection. In general, since HBV is usually transmitted through blood, it is recommended that the mothers to consider nipple care during breastfeeding to ensure the closure of the nipple and avoid bleeding (27). Meanwhile, when the infants have the gastrointestinal mucosal injury, breast-feeding should be considered carefully.
3.2. Antiviral Therapy in Preventing MTCT of HBV
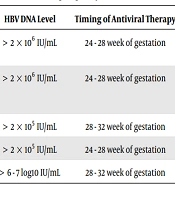
Considering these mechanisms, passive-active prophylaxis after birth can prevent transmission during delivery or in the postpartum period, but it has no effect on the intrauterine route of transmission. Other strategies have been tested in this setting, including antiviral drugs, pregnant women injected with hepatitis B immunoglobulin (HBIG) and mode of delivery (28). The MTCT rate decreased from 90% to 10% due to the administration of passive-active immunoprophylaxis that injected HBIG within 24 h after birth (preferably within 12 hours) and injected 10ug HB vaccine at a different anatomical site. In addition, 10 ug HB vaccine was administered to the infants aged 1 and 6 months (6, 29-32). Studies have shown that the infants born to mothers with high viral load are more likely to be infected with HBV (33, 34). Therefore, domestic and foreign guidelines recommend orally taking antiviral drugs during pregnancy to reduce the rate of MTCT (Table 1). The efficacy and safety of LAM and LDT in preventing MTCT of HBV were appropriate before tenofovir disoproxil fumarate (TDF) went public (35-37). However, there are controversies regarding timing of initiation of antiviral therapy, HBV DNA levels at the onset of antiviral therapy, postpartum discontinuation time, etc.
| HBV DNA Level | Timing of Antiviral Therapy | Timing of Discontinuation | Antiviral Drugs | |
|---|---|---|---|---|
| Consensus on clinical management of hepatitis B virus-infected women of childbearing age (38) | > 2 × 106 IU/mL | 24 - 28 week of gestation | Postpartum 1 - 3 months | TDF/LDT |
| Management algorithm for interrupting mother-to-child transmission of hepatitis B virus (39) | > 2 × 106 IU/mL | 24 - 28 week of gestation | Postpartum 1 - 3 months | TDF/LDT |
| 2018 AASLD (4) | > 2 × 105 IU/mL | 28 - 32 week of gestation | Postpartum 1 - 3 months | TDF |
| 2017 EASL (40) | > 2 × 105 IU/mL | 24 - 28 week of gestation | Up to 12 weeks after delivery | TDF |
| 2015 APASL (41) | > 6 - 7 log10 IU/mL | 28 - 32 week of gestation | At delivery | TDF/LDT |
It is controversial to consider timing of initiation of antiviral therapy in pregnant women with high viral load in the second trimester or the third trimester of pregnancy. However, most studies suggest that there is no difference in the rate of blocking MTCT, regardless of the antiviral treatment during the second or third trimester of pregnancy. Pan et al. (42) retrospectively enrolled 249 mothers with HBV DNA > 6 log10 copies/mL who received LAM during pregnancy and 66 and 94 subjects received LAM during the second and third trimesters, respectively. They found that LAM treatment initiated in the second or third trimester for mothers with HBV DNA levels below 9log10 copies/mL, was equally safe and effective in preventing vertical transmission. Tan et al. (43) enrolled pregnant women positive for HBsAg who began LDT treatment before 14 weeks of gestation (early), between 14 and 28 weeks of gestation (late), or not at all (control). HBV MTCT rates in the early and late treatment and also in the control groups were 0, 0, and 4.69%, respectively. Sun et al. (44) conducted a study that comprised pregnant women with CHB, with HBV DNA ≥ 1.0 × 107copies/mL as well as the increased alanine aminotransferase (ALT) levels. Groups A (n = 62) and B (n = 61) were treated with LDT initiated at 12 weeks or 20 - 28 weeks after gestation, respectively. No infants in groups A and B were HBsAg-positive, so they concluded that administration of LDT to HBV-infected mothers, started during early and middle pregnancy, completely blocked MTCT.
TDF is a nucleotide analogue and a potent inhibitor of HBV polymerase (45-47). After 6 years of monotherapy for CHB, no drug resistance was found and TDF is also currently the preferred drug for preventing MTCT of HBV (48). Recent studies on the efficacy and safety of TDF in blocking MTCT during pregnancy are listed in Table 2. The rate of MTCT in TDF group was significantly higher than that of the control group, regardless of whether oral TDF was administered in the second or third trimester of pregnancy, however there was no study on the comparison between the second trimester and third trimester groups. The study published in the New England Journal of Medicine in 2018 by Thai scholars suggested that there was no significant difference in the MTCT rate between the TDF and control groups, but the MTCT rate in TDF group was 0 and the sample size was small (5). By enrolling more pregnant women, they probably obtained different results. The maternal and infant safety profiles were similar in the TDF and control groups. However, the long-term safety of infants born to mothers who used TDF during pregnancy needs further investigations (49).
| First Author | Publish Date | Sample Size | Inclusion Criteria | Trial Design | Efficacy | Safety |
|---|---|---|---|---|---|---|
| Chen (50) | 2015 | 118 | HBsAg and HBeAg-positive, HBV DNA > 7.5log10 IU/mL | The mothers received no medication or 300 mg TDF daily from 30 - 32 weeks of gestation until 1 month postpartum. | Of the newborns, the TDF group had a lower rate of HBV DNA positivity at 6 months (P = 0.0481). | Maternal creatinine and CK levels, rates of congenital anomaly, premature birth, and growth parameters in infants were comparable in both groups. |
| Pan (51) | 2016 | 200 | HBsAg and HBeAg-positive, HBV DNA > 2 × 105 IU/mL | All participants were randomly assigned in a 1:1 ratio, to receive usual care without antiviral therapy or to receive TDF from 30 - 32 weeks of gestation until postpartum week 4. | Both in the intention-to-treat analysis (P = 0.007) and the per-protocol analysis (P = 0.01), at postpartum week 28, the rate of MTCT was significantly lower in the TDF group than that of the control group. | The maternal and infant safety profiles were similar in the TDF and the control groups, including birth-defect rates (2% and 1%; P = 1.00) |
| Wan | 2017 | 116 | HBsAg-positive, HBV DNA > 1 × 106 copeis/mL | Mothers were divided into the observation (orally taking TDF from the 28th week of gestation until the end of pregnancy) and control groups (no antiviral treatment). | The positive rate of neonatal HBsAg in the observation group (4.05%) was significantly lower than that of the control group (16.70%, P < 0.05). | The incidence of adverse reactions was low (5.41%). These adverse reactions were mild and improved after symptomatic treatment |
| Jourdain (5) | 2018 | 331 | HBsAg and HBeAg-positive, ALT < 60U/L | Mothers received TDF or placebo from 28 weeks of gestation to 2 months postpartum. | None of the infants in the TDF group and 3 infants in the placebo group had HBV infection at 6 months (P = 0.12). | There is no significant difference between two groups in adverse event of grade 3 or 4 or a serious adverse event of maternal and infants (P = 0.61) |
Different opinions are provided by different countries in their guidelines regarding the level of HBV DNA to initiate antiviral therapy. Zou et al. (52) conducted a study that demonstrated HBV immunoprophylaxis failure occurred among infants born to HBeAg-positive mothers with HBV DNA levels > 6log10copies/mL. No immunoprophylaxis failure occurred in infants born to the mothers who were HBeAg-negative or had HBV DNA levels < 6log10 copies/mL. The HBV DNA level to initiate antiviral therapy in the 2017 EASL guidelines and 2018 AASLD guidelines were referred to these studies. Liu et al. (53) enrolled 256 mother-child pairs with positive maternal HBsAg and they found that additional treatment strategies should be considered in HBeAg-positive mothers with an HBV DNA level above 6 - 7log10IU/mL. Korean scholars analyzed the cost‑effectiveness of antiviral prophylaxis during pregnancy and they found that it is advisable to augment the current national Perinatal Hepatitis B Prevention Program in Korea to provide antiviral therapy to women with HBV DNA ≥ 106 copies/mL during their late pregnancy (54). However, the consensus on clinical management of HBV-infected women of childbearing age suggested that pregnant women with HBV infection, started antiviral therapy at 24 - 28 weeks of gestation with HBV DNA level > 2 × 106 IU/mL (38). The management algorithm for interrupting MTCT of HBV recommended that when HBV DNA level is greater than 2 × 106 IU/mL, antiviral treatment can be done with either TDF or LdT (39).
The time of antiviral therapy discontinuation for blocking MTCT is also controversial. The main concern is the liver dysfunction after discontinuation. Most pregnant women tend to have increased ALT after the discontinuation of antiviral drugs during pregnancy (50). Therefore, more and more scholars are devoted to explore the optimal time for discontinuation to minimize the incidence of postpartum liver dysfunction. Nguyen et al. (55) found liver dysfunction common after delivery and most of cases were recovered by themselves. Continuing oral antiviral drugs could not reduce the risk of liver dysfunction. Therefore, it is suggested that preventive antiviral therapy in pregnant women with immune tolerance should be discontinued after blocking MTCT. ter Borg et al. (56) conducted a study on 38 pregnant women. It was found that the liver dysfunction in pregnant women before delivery was about 0.8 × ULN, and the proportion of liver dysfunction after delivery was as high as 62%, increasing to 1.6 × ULN, and the highest ALT level was 4.3 × ULN. However, the mechanism of liver dysfunction in pregnant women after delivery is controversial. It may be due to a series of changes in endocrine and immune system during pregnancy, which leads to the suppression of immune function and the rapid recovery of postpartum adrenocortical hormone levels. As with the activation of HBV after discontinuation of corticosteroid therapy, postpartum liver function is abnormal. At the same time, the mother's estrogen and progesterone levels drop rapidly after delivery, the cellular immune function recovers rapidly and induces the immune clearance of HBV, leading to postpartum liver dysfunction (57-60). Although the flares are often mild and resolved spontaneously, cases of acute liver failure have been described in the peripartum period (25, 55, 61).
4. Conclusions
In conclusion, the mechanisms of MTCT of HBV remain unclear and further studies are needed. Domestic and foreign guidelines recommend that pregnant women with high viral load take orally TDF in the second trimester or third trimester of pregnancy to block MTCT of HBV. The effectiveness and safety of this measure have been proved. Preventive antiviral therapy is recommended for discontinuing the drug at the latest 3 months after delivery. Most pregnant women tend to suffer from the increased ALT after the discontinuation of antiviral drugs during pregnancy. Therefore, close ALT levels monitoring after drug discontinuation is essential. Strategies for further improvements in blocking MTCT may include post-immunization testing for high-risk children, re-immunization strategies for children with low or no response, development of new hepatitis B vaccine, etc.