1. Background
Chronic hepatitis B (CHB) is a prevalent disease worldwide. In 2010, about 248 million people around the world were positive for hepatitis B surface antigen (HBsAg). The prevalence rate was approximately 5.49% in China (approximately 93 million) (1, 2). Hepatitis B-related complications cause 600,000 deaths per year (3).
For high-risk populations, such as patients with hemophilia, being infected with hepatitis B virus (HBV) via blood transfusion or drug abuse by sharing needles, the prognosis gets worse after HBV infection (4). It is recommended that patients with CHB should undergo anti-virus treatment if needed. Furthermore, CHB patients with decompensated liver cirrhosis should undertake the treatment to prevent hepatitis B recurrence after liver transplantation (5). Non-alcoholic fatty liver disease (NAFLD) is caused by genetic susceptibility factors, excess nutrients, and related complications. Based on recent guidelines, it is defined by the presence of ≥ 5% hepatic steatosis with or without hepatocellular injury (6). Hepatic steatosis is common among CHB patients, and its prevalence fluctuates between 14% and 70%; the proportion of non-drinking HBV-infected patients was 25.6% (7). Most previous studies suggested that hepatic steatosis in CHB patients was primarily associated with metabolic factors (8).
There are many different ways to evaluate the severity NAFLD, which include liver biopsy, serum markers, transient elastography, magnetic resonance imaging, and so on (9, 10). Nevertheless, histology remains the gold standard despite limitations due to sampling variability (6).
As we all know, hepatic fibrosis is an important factor affecting the prognosis of liver diseases. However, the relationship between the severity of NAFLD and liver fibrosis in CHB patients remains controversial (11-13). Previous work suggests that combination with steatosis was an independent risk factor for the development of liver cirrhosis in patients with chronic hepatitis B (13). By contrast, another study suggested that steatosis might be a protective factor in patients with CHB (14). Moreover, much of the literature supports the view that there is no correlation between them (15). Nevertheless, until recently, there has been little information available on the relationship between steatohepatitis in CHB patients and the severity of fibrosis.
2. Objectives
The current research aimed to investigate the association between the severity of steatosis or steatohepatitis and liver fibrosis, assessed by the METAVIR system in Chinese CHB patients.
3. Methods
This study was approved by the Human Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University. The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
3.1. Patients
The researchers retrospectively examined 570 consecutive in-patients undergoing liver biopsy from December 2009 to March 2018 at the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. The study included patients aged 18 years or above and under 65 years, who had positive results for hepatitis B surface antigen for at least six months. Furthermore, NAFLD patients without HBV infection with the same age range were included. Both treatment-naive and on-treatment CHB patients were recruited. The exclusion criteria were as follows: (i) co-infection with other viruses, including hepatotropic virus (hepatitis A, C, D, E), human immunodeficiency virus, cytomegalovirus, EB virus, and others; (ii) autoimmune liver disease, primary sclerosing cholangitis, primary biliary cholangitis, Wilson’s disease, hemochromatosis, or 1-antitrypsin deficiency; (iii) alcoholic liver disease; (iv) previous and/or current intake of steatosis-inducing drugs (including corticosteroids, methotrexate, and tamoxifen) or potentially hepatotoxic drugs, evaluated by an interview; (v) Buga syndrome, parasite-related liver damage, hyperthyroidism; currently active or suspected hepatocellular carcinoma or other malignant diseases; previous liver transplantation; and current pregnancy.
3.2. Methods
3.2.1. Bio-Clinical Assessment
The study data were collected within four weeks of liver biopsy. Demographic parameters and routine blood tests, such as liver function tests, metabolic parameters and viral markers, were all collected.
3.2.2. Histological Data
Liver specimens were obtained using a 16- or 18-G needle (Bard Magnum, GA, USA). Specimens with fewer than six portal tracts were excluded. Liver fibrosis was staged according to the METAVIR scoring system (16). The NAS scoring system (0 to 8) was used to evaluate hepatic inflammation in the NAFLD patients with or without CHB (17).
3.2.3. Statistical Analysis
Statistical analysis was performed using IBM SPSS 20.0 (IBM Corp, NY, USA). Continuous variables were expressed as mean ± standard deviation or median (25th to 75th percentiles), as appropriate. Qualitative data were presented as numbers and percentages. Student’s t-test was used for the parametric test and Mann-Whitney U test was used for the non-parametric test. For categorical variables, the researchers used the chi-squared or Fisher exact tests. The Kruskal-Wallis H test was used for correlation of hepatic fibrosis severity and the severity of steatosis or NAS score. A P value of < 0.05 (two-tailed) was considered statistically significant.
4. Results
The enrollment strategy is depicted in Figure 1. The demographic and laboratory characteristics of the CHB patients and NAFLD patients are shown in Tables 1 and 2. Body mass index, gamma-glutamyl transferase (GGT), cholinesterase, fiber bragg grating (FBG), uric acid, triglyceride, high density lipoprotein (HDL), apolipoprotein (APOB), and hemoglobin were significantly different between CHB patients with and without NAFLD. However, only BMI, aspartate aminotransferase (AST), alanine aminotransferase (ALT), GGT, and uric acid showed a significant difference between NAFLD patients with NAS ≥ 5 and NAS < 5, among CHB patients (Table 1).
| CHB (N = 272) | NAFLD (N = 31) | CHB Without NAFLD (N = 164) | CHB with NAFLD (N = 108) | P Valueb | NAS < 5 of 108 (N = 79) | NAS ≥ 5 of 108 (N = 29) | P Valuec | |
|---|---|---|---|---|---|---|---|---|
| Age | 38.4 ± 8.03 | 34.55 ± 13.43 | 38.33 ± 8.04 | 38.50 ± .8.04 | 0.86 | 38.53 ± 8.42 | 38.41 ± 7.05 | 0.95 |
| Male gender | 223 (82.0) | 26 (83.9) | 126 (76.8) | 97 (89.8) | < 0.01 | 70 (88.6) | 27 (93.1) | 0.72 |
| BMI, kg/m2 | 24.31 ± 3.08 | 26.39 ± 3.41 | 23.27 ± 2.74 | 25.89 ± 2.92 | < 0.01 | 25.34 ± 2.58 | 27.39 ± 3.30 | < 0.01 |
| < 18.5 | 9 (3.3) | 0 | 9 (5.5) | 0 | 0 | 0 | ||
| 18.5 - 25 | 162 (59.6) | 10 (32.3) | 113 (68.9) | 49 (45.4) | 42 (53.2) | 7 (24.1) | ||
| ≥ 25 | 101 (37.1) | 21 (67.7) | 42 (25.6) | 59 (54.6) | 37 (46.8) | 22 (75.9) | ||
| AST, U/L | 29.5 (25 - 43.75) | 54.0 (36 - 86) | 29 (24 - 45.5) | 30 (25 - 39) | 0.90 | 29 (24 - 34) | 37 (26 - 74) | < 0.01 |
| ALT, U/L | 40 (29 - 63) | 112 (49 - 183) | 37.5 (28 - 65) | 45 (33.25 - 63) | 0.06 | 41 (32 - 54) | 56 (40 - 109) | < 0.01 |
| Total bilirubin, µmol/L | 13.05(9.92 - 16.4) | 11.9 (8.5 - 16.6) | 13.45 (10.42 - 17.58) | 12.1 (9.7 - 15.48) | 0.07 | 12(9.4 - 15.3) | 12.1 (10.6 - 16.25) | 0.26 |
| Albumin, g/L | 44.74 ± 3.61 | 47.44 ± 3.53 | 44.45 ± 3.71 | 45.18 ± 3.44 | 0.10 | 45.30 ± 3.49 | 44.85 ± 3.33 | 0.55 |
| GGT, U/L | 32 (22 - 49) | 96 (51 - 193) | 27 (20 - 44) | 38 (29 - 59.5) | < 0.01 | 33 (25 - 47) | 49 (35.5 - 88.5) | < 0.01 |
| Cholinesterase, U/L | 8763.02 ± 2050.73 | 10305.35 ± 1693.60 | 8418.24 ± 2166.29 | 9286.58 ± 1744.59 | < 0.01 | 9290.82 ± 1725.70 | 9275.03 ± 1826.10 | 0.97 |
| FBG, mmol/L | 5.37 ± 1.21 | 5.76 ± 1.58 | 5.16 ± 1.09 | 5.68 ± 1.32 | < 0.01 | 5.57 ± 1.24 | 5.98 ± 1.52 | 0.16 |
| Uric acid, mmol/L | 383.82 ± 92.86 | 464.55 ± 113.54 | 364.26 ± 84.29 | 413.52 ± 97.67 | < 0.01 | 399.39 ± 89.64 | 452.03 ± 109.40 | 0.01 |
| Cholesterol, mmol/L | 4.86 ± 0.94 | 5.04 ± 1.06 | 4.84 ± 0.96 | 4.89 ± 0.93 | 0.69 | 4.91 ± 1.00 | 4.84 ± 0.72 | 0.75 |
| Triglyceride, mmol/L | 1.38 ± 0.84 | 2.09 ± 1.60 | 1.26 ± 0.80 | 1.57 ± 0.86 | < 0.01 | 1.55 ± 0.82 | 1.62 ± 0.96 | 0.69 |
| HDL, mmol/L | 1.22 ± 0.30 | 1.14 ± 0.19 | 1.26 ± 0.33 | 1.15 ± 0.24 | < 0.01 | 1.15 ± 0.26 | 1.15 ± 0.19 | 0.95 |
| LDL, mmol/L | 3.15 ± 0.85 | 3.32 ± 0.96 | 3.10 ± 0.85 | 3.24 ± 0.84 | 0.16 | 3.26 ± 0.88 | 3.21 ± 0.75 | 0.78 |
| APOA, g/L | 1.4 ± 0.20 | 1.38 ± 0.17 | 1.41 ± 0.22 | 1.40 ± 0.19 | 0.80 | 1.41 ± 0.19 | 1.37 ± 0.18 | 0.34 |
| APOB, g/L | 1.056 ± 0.29 | 1.19 ± 0.35 | 1.02 ± 0.28 | 1.12 ± 0.28 | < 0.01 | 1.11 ± 0.28 | 1.14 ± 0.29 | 0.60 |
| Platelet, 109 /L | 203.82 ± 55.00 | 236.81 ± 63.75 | 203.84 ± 51.92 | 203.79 ± 59.61 | 0.99 | 210.25 ± 57.58 | 186.21 ± 62.50 | 0.06 |
| Hemoglobin, g/L | 146.00 ± 14.75 | 150.29 ± 10.74 | 144.22 ± 16.14 | 151.14 ± 11.19 | < 0.01 | 150.72 ± 10.92 | 152.28 ± 12.02 | 0.53 |
| Antiviral therapy | 65 (31.4) | 0 | 36 (22.0) | 29 (26.9) | 0.35 | 20 (25.3) | 9(31.0) | 0.55 |
| HBeAg +, % | 110 (40.4) | 0 | 71 (43.3) | 39 (36.1) | 0.24 | 28 (35.4) | 11 (37.9) | 0.82 |
| HBV-DNA, log IU/mL | 5.23 (3.34 - 6.95) | 0 | 5.40 (3.59 - 7.46) | 4.84 (2.92 - 6.50) | 0.07 | 4.74 (3.37 - 6.50) | 4.96 (< 1.3 - 6.67) | 0.48 |
Abbreviations: ALT, alanine aminotransferase; APOA, apolipoprotein A1; APOB, apolipoprotein B100; AST, aspartate aminotransferase; BMI, body mass index; CHB, chronic hepatitis B; FBG, fast blood-glucose; GGT, gamma-glutamyl transferase; HBeAg, hepatitis B e antigen; HBV-DNA, hepatitis B virus DNA; HDL, high-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; LDL, low-density lipoprotein.
aValues are expressed as the mean ± standard deviation, median (25th - 75th), or No. (%).
bBetween the CHB with and without NAFLD groups.
cBetween NAS ≥ 5 or NAS < 5 in CHB with NAFLD.
| Variable | CHB (N = 272) | NAFLD (N = 31) | CHB with NAFLD (N = 108) | CHB Without NAFLD (N = 164) |
|---|---|---|---|---|
| Fibrosis | ||||
| 0 | 27 (10.0) | 8 (25.8) | 7 (6.5) | 20 (12.2) |
| 1 | 83 (30.5) | 6 (19.3) | 36 (33.3) | 47 (28.7) |
| 2 | 98 (36.0) | 14 (45.2) | 34 (31.5) | 64 (39.0) |
| 3 | 36 (13.2) | 1 (3.2) | 17 (15.7) | 19 (11.6) |
| 4 | 28 (10.3) | 2 (6.5) | 14 (13.0) | 14 (8.5) |
| Steatosis | ||||
| < 5% | 164 (60.3) | |||
| 5% - 33% | 85 (31.3) | 11 (35.5) | 85 (78.7) | |
| 34% - 66% | 18 (6.6) | 9 (29.0) | 18 (16.7) | |
| > 66% | 5 (1.8) | 11 (35.5) | 5 (4.6) | |
| NAS | ||||
| 0 - 2 | 3 (9.7) | 19 (17.6) | ||
| 3 - 4 | 5 (16.1) | 60 (55.5) | ||
| ≥ 5 | 23 (74.2) | 29 (26.9) |
Abbreviations: CHB, chronic hepatitis B; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score.
aValues are expressed as the No. (%).
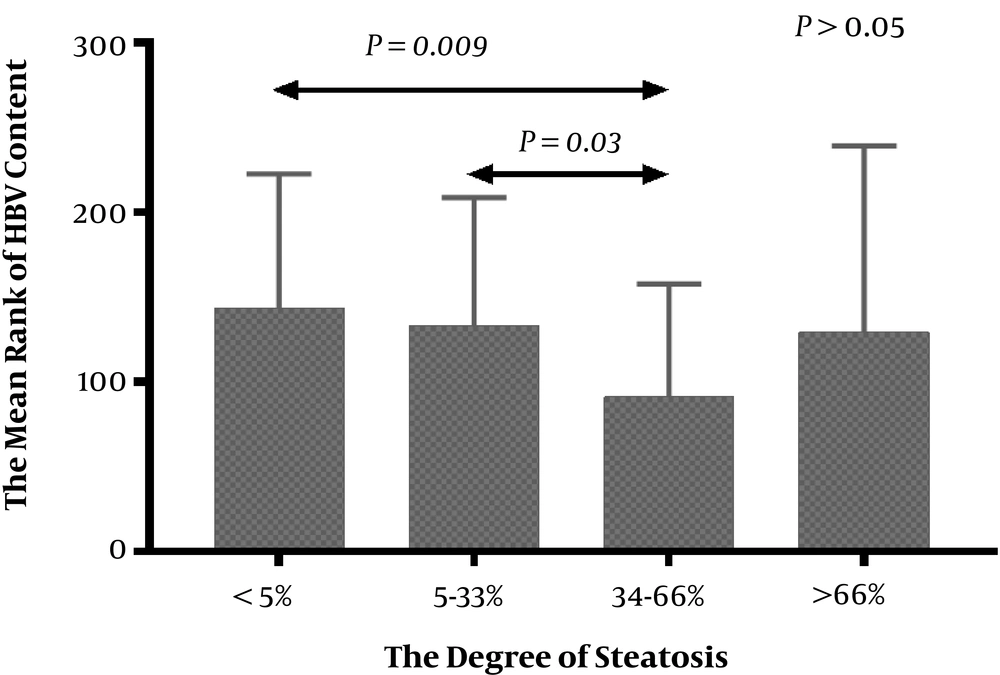
The correlation of steatosis and HBV DNA level in CHB patients is showed in Figure 2. In general, the severity of liver steatosis was not associated with HBV DNA level in CHB patients with or without NAFLD (P > 0.05). However, the HBV DNA content of patients with steatosis 34-66% was significantly less than patients with less fat content or without steatosis.
4.1. Pathological Findings
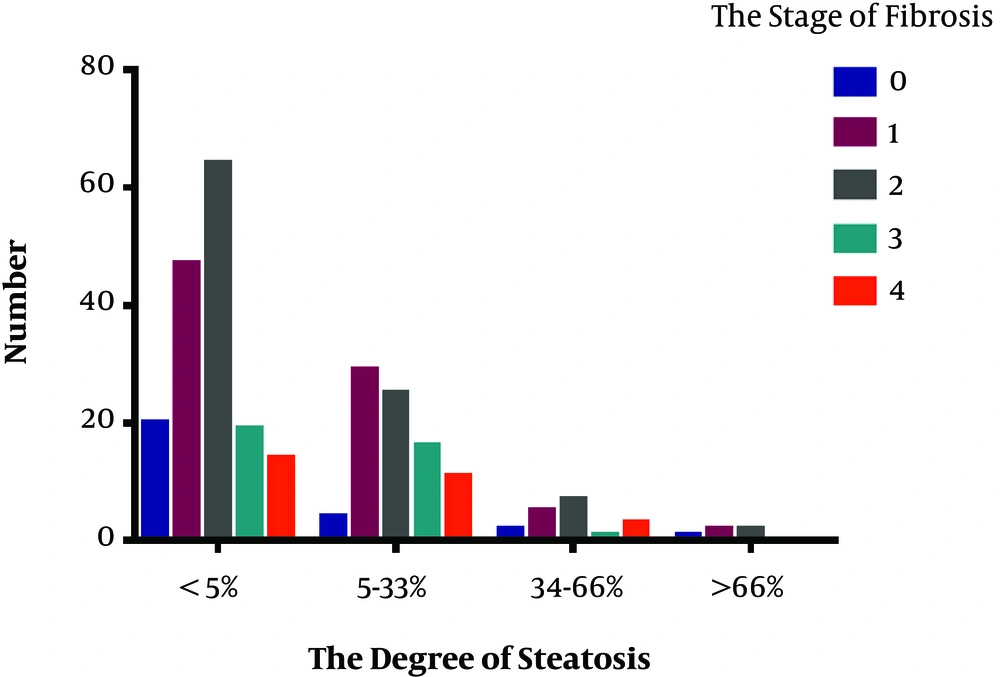
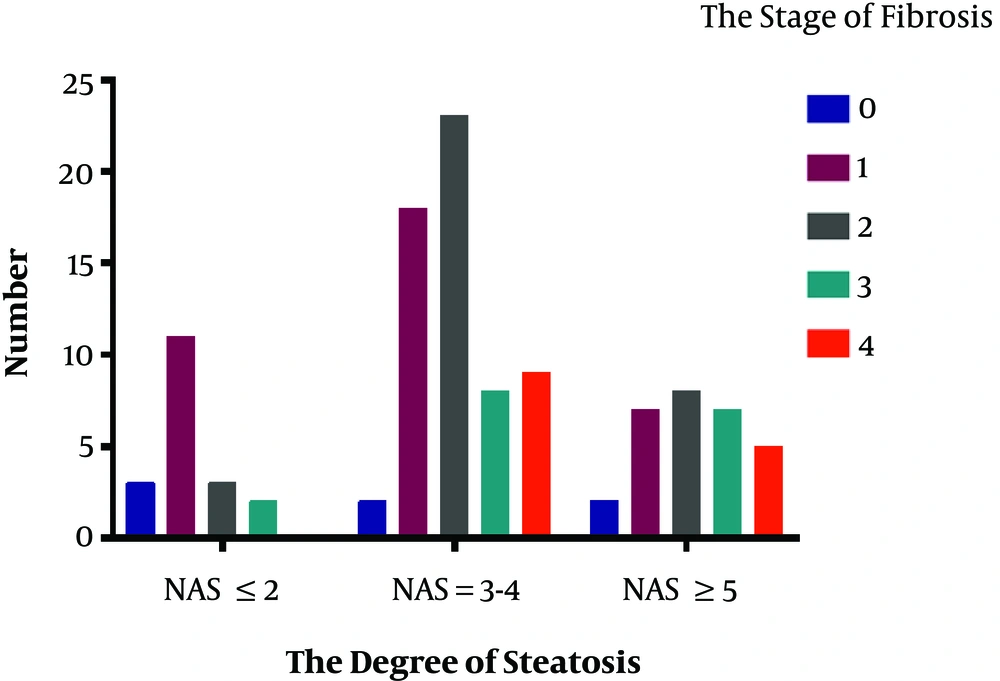
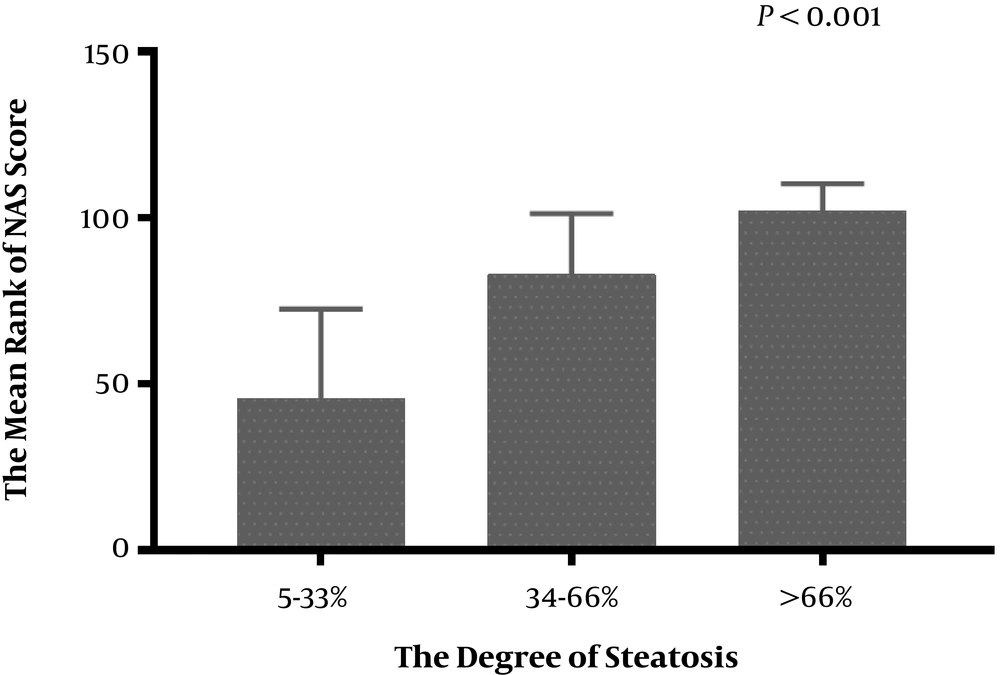
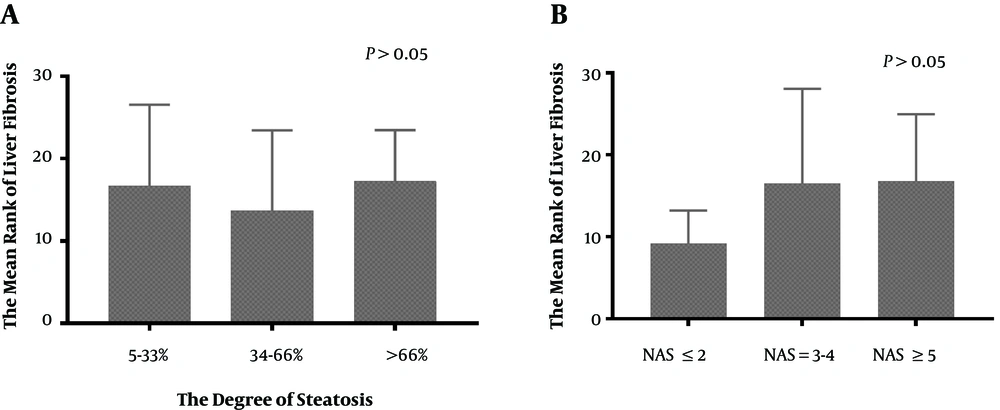
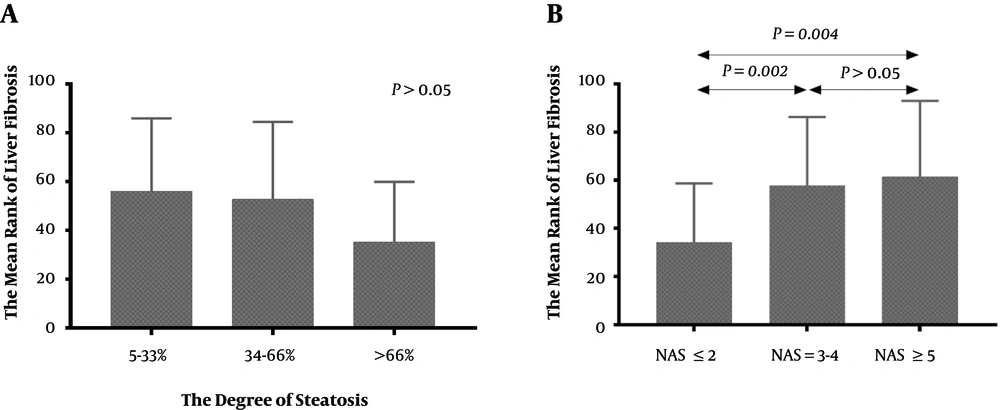
The results of liver pathology of the 272 CHB patients is showed in Figures 3 and 4. This research analyzed the association between the severity of hepatic steatosis and NAS score in 108 CHB with NAFLD patients; there was a correlation between them (P < 0.001) (Figure 5). The NAS score tended to increase with increasing degree of steatosis in CHB patients. As shown in Figure 6A and B, the severity of liver fibrosis was not associated with steatosis nor NAS score in NAFLD patients without CHB. Similarly, the results were suggestive of no significant difference in the severity of liver fibrosis in CHB patients with varying degrees of steatosis (P > 0.05) (Figure 7A). Interestingly, the severity of liver fibrosis increased with the NAS score (P = 0.004) in CHB patients with NAFLD. As shown in Figure 7B, hepatic fibrosis in CHB patients with NAS = 3 - 4 (P = 0.002) and NAS ≥ 5 (P = 0.004) were more severe than in those with NAS ≤ 2, although the difference in liver fibrosis severity between the NAS = 3 - 4 and NAS ≥5 groups was not statistically significant (P > 0.05).
5. Discussion
This research enrolled 272 CHB patients and 31 NAFLD patients without CHB undergoing liver biopsy; the prevalence of the steatosis in CHB patients was similar to that reported in a related study (18). The results were consistent with those of previous studies that suggested hepatic steatosis was related to host metabolic factors yet not to viral factors (11). Although one experimental study revealed that over-expression of HBx (hepatitis B X) protein induced hepatic lipid accumulation in HepG2 and HBx-transgenic mice (19).
Furthermore, as it was shown above that there were no significant associations between the fat content nor NAS score and liver fibrosis severity in NAFLD patients without CHB. Likewise, this research found that the fat content was not related to the severity of liver fibrosis in CHB patients with NAFLD, consistent with the findings of previous studies (20). According to previous work, most experts held the view that host factors were responsible for intra-hepatic fat accumulation in CHB patients (21).
Analyzed from another aspect, in NAFLD patients with the progression of steatohepatitis, it is widely believed that once cirrhosis develops, the fat content in the liver decreases rapidly without significant weight loss (22). Accordingly, CHB patients with advanced fibrosis or cirrhosis may have a similar situation. In other words, the disappearance of steatosis could be the consequence but not the cause of fibrosis, since the mechanism of “burned out” NAFLD in cirrhosis was not clear in NAFLD itself. This is why further study is expected to explore the mechanisms of the paradoxical loss of steatosis with cirrhosis in CHB with NAFLD patients and NAFLD itself.
Interestingly, the severity of liver fibrosis was related to NAS score in CHB patients with NAFLD. To some extent, this result was supported by a recent study involving 1606 patients in Hong Kong (12), which demonstrated that severe steatosis measured by controlled attenuation parameter (CAP) was associated with severe fibrosis in CHB patients. Meanwhile, the current study showed that the severity of steatosis was closely associated with the NAS score in CHB patients. As the results indicated, there was no significant difference between NAS = 3 - 4 and NAS ≥ 5, since NAS = 3 - 4 is defined as “borderline non-alcoholic steatohepatitis (NASH)” in NAFLD patients (23), which is not a good cutoff point to distinguish from NASH with NAS ≥ 5. However, it is because NAS = 3 - 4 cannot rule out the existence of NASH that similar severity of liver fibrosis between NAS = 3 - 4 and NAS ≥ 5 instead supported our conclusion. Accordingly, it could be suggested that a more severe steatohepatitis caused by NAFLD in chronic hepatitis B is associated with more severe liver fibrosis.
Compared with previous studies, there were two main strengths in the current work. First, the researchers assessed the severity of NAFLD and fibrosis, using liver pathology; the results were more authentic and reliable. Second, previous studies illustrated little about steatohepatitis in CHB patients with fibrosis, while the current work might provide some reference value. Of course, this study had several limitations. First, this was a retrospective study, thus it was not possible to determine a causal relationship between NAS score and the severity of fibrosis. A prospective study with repeated liver biopsy may be better for elucidating the relationship between the two. Second, due to the limitation of sample size in a single center, further multi-center prospective studies are expected to obtain more accurate and reliable results.
In conclusion, this study indicated that hepatic steatosis in CHB patients was primarily related to metabolic factors and not to viral markers, and that the severity of fibrosis was significantly associated with NAS score, yet not with fat content.