1. Background
Chronic hepatitis C virus (HCV) infection affects 71 million patients (1), remaining one of the most important public health problems worldwide. Although interferon-free combinations of direct-acting antivirals generate very high rates of sustained virologic response, patients with advanced fibrosis and cirrhosis remain at risk for the development of hepatocellular carcinoma (HCC), especially in the absence of fibrosis regression after successful therapy (2). Several epigenetic mechanisms, including noncoding microRNAs (miRNAs), are studied in order to understand the pathogenic mechanisms involved in the setting and progression of liver fibrosis and carcinogenesis (3). HCV infection modulates a series of cellular microRNAs, miR-122 as a classic example, proving to be essential during HCV replicative cycle (4). Other miRNAs abundantly present in the liver, such as miR-125b as a highly conserved homolog of lin-4, are studied for their roles in major cellular pathways including inflammatory response, fibrogenesis, and hepatocellular oncogenesis (5, 6). The exosomal miR-125b level was correlated with the prognosis of hepatocellular carcinoma (7) and recently, plasma levels of miR-125b have been proposed as a non-invasive biomarker in chronic viral hepatitis (8), HBV-induced cirrhosis, and HBV-associated hepatocellular carcinoma (9).
2. Objectives
The present study aimed to investigate the potential value of miR-125b as a non-invasive biomarker in chronic hepatitis C and to evaluate the correlation between miR-125b expression and viral or host factors involved in the progression of liver disease.
3. Methods
An observational, retrospective study was conducted on 94 treatment-naïve HCV-infected patients [mean age 49.8 ± 11.5 years; range 30-68 years, 59.6% females]. The study was approved by the Ethics Committee of the Institute of Virology.
MiR-125b plasma expression was analyzed by real-time PCR with TaqMan® MicroRNA Assay (Applied Biosystems, USA), according to the manufacturer’s protocol. Briefly, miRNA was isolated from 300 µL of plasma using NucleoSpin® miRNA Plasma Kit (Macherey-Nagel, Germany). Reverse-transcription was performed using TaqMan® MicroRNA reverse transcription kit (Thermo Fisher Scientific, USA). The reverse-transcripted DNA fragments, resuspended in Hi-Di formamide, were amplified together with a DNA internal sizing standard in an ABI 7300 Genetic Analyzer. miRNA expression was normalized against those of an endogenous control: Cellular miR-39 (Spike-In Cel-miR-39, Qiagen, USA) and against those of healthy volunteers, and determined by the 2-ΔΔCt method: ΔCt = Ct miR-125b - Ct Cel-miR-39.
Serum HCV viral load was quantified using the RT-PCR-Cobas TaqMan HCV test, V2.0 (Roche Molecular Systems, Branchburg, NJ, USA), with a linear range of HCV-RNA between 15 and 100.000.000 IU/mL, and a lower limit of sensitivity of 15 IU/mL.
Liver fibrosis was evaluated using a noninvasive method -transient elastography (TE, FibroScan, Echosens, France)- with good accuracy in differentiating mild/moderate fibrosis (corresponding to Metavir score F1/F2) from advanced fibrosis (F3/F4) at a cutoff of 7.1 kPa for livers stiffness (10).
Polymorphism in the IL28B gene (SNP on chromosome 19-rs12979860) was tested with Custom TaqMan 5'-allelic discrimination assay (Applied Biosystems, USA).
The transferases levels were measured with the spectrophotometric standardized methods of the International Federation for Clinical Chemistry. The upper normal limit was 50 IU/L for Alanine Aminotransferase (ALT); for gamma-glutamyl transferase (GGT), it was 61 IU/L in men and 36 IU/L in women.
Serum alpha-fetoprotein (AFP) levels were measured using electrochemiluminescence (ECLIA, Roche Diagnostics), with normal values defined as less than 10 ng/mL.
Statistical analysis was done using SPSS V.25 (Chicago, USA), employing Spearman rank correlation test for continuous variables and the Mann Whitney U test for differences between various groups. A P value of less than 0.05 was considered statistically significant.
4. Results
All patients were infected with HCV genotype 1b and had active viral replication (mean HCV viral load = 6.1 ± 1.4 log10 IU/mL). 55.3% had high baseline HCV viral loads (above 5.8 log10 IU/mL). The favorable CC IL28B polymorphism was present in only 21.3% of the patients, while 60.6% had the heterozygous CT genotype and the rest had the unfavorable TT polymorphism. Significant cytolysis, with serum ALT values 1.5 times greater than the upper limit of normal, was present in 42.6% of the patients; only had 27.7% normal ALT. Increased serum GGT values suggestive of cholestasis were present in 73.4% of the patients. Only had 11.7% of the patients high Alpha-fetoprotein serum values (AFP > 10 ng/mL).
Advanced fibrosis, defined by a TE cutoff value of ≥ 7.1 kPa for liver stiffness, was present in 61.7% of the patients, while the rest had mild/moderate fibrosis and none had cirrhosis.
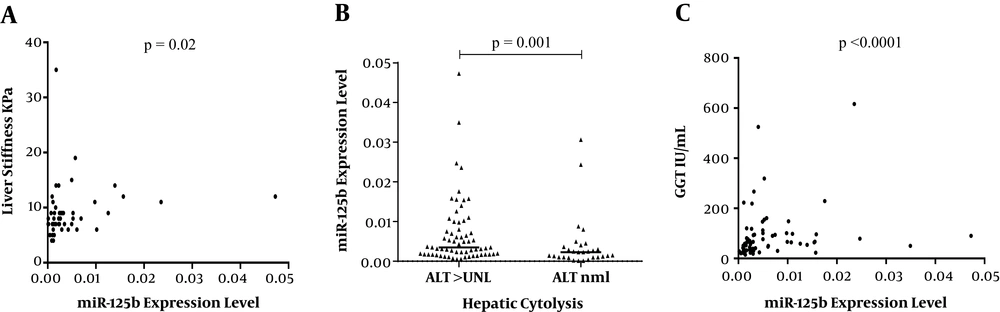
In a univariate analysis, an up-regulated expression of miR-125b was found in plasma samples of patients with advanced liver fibrosis as compared to ones with mild/moderate fibrosis [mean miR-125b value = 0.002 (range 0.0001 - 0.04) versus 0.001 (range 0.0001-0.01) (P = 0.02)] and it was directly correlated with the degree of hepatic cytolysis (increased serum ALT values, P = 0.001) and cholestasis (increased GGT values, P < 0.0001), (Figure 1). No significant associations were found between miR-125b expression and other important viral and host predictors for liver disease evolution: High HCV viral load of > 5.8 log10 IU/mL (P = 0.56), increased alpha-fetoprotein levels (P = 0.27), unfavourable IL28B genotype (P = 0.5), patient’s gender (P = 0.13), or age (P = 0.5).
In a multiple regression analysis, an upregulated miR-125b expression remained independently associated only with advanced fibrosis and GGT levels, but not with serum ALT (P = 0.026; R2 = 0.242) (Table 1).
| Dependent Variable: miR125b | Std. Error | P | 95% Confidence Interval | |
|---|---|---|---|---|
| Fibrosis | 0.002 | 0.03 | 0.0004 | 0.008 |
| Serum GGT | 0.00002 | 0.05 | - 0.000005 | 0.00005 |
| Serum ALT | 0.000004 | 0.49 | - 0.00001 | 0.000006 |
5. Discussion
The present study reported a direct correlation between an up-regulated plasma miR-125b expression and advanced liver fibrosis in treatment-naïve HCV infected patients. This correlation was independent of the viral replication level and was not influenced by patients age, gender, or IL28 genotype as known predictors of the natural and on-treatment evolution of hepatitis C (11). It will be interesting to study the miR-125b expression in patients successfully treated with direct-acting antiviral regimens, as previous studies showed that miR-125b expression in peripheral blood mononuclear cells was significantly predictive of sustained virological response in interferon-treated HCV infected patients, again irrespective of interleukin-28B genotype, age, or gender (12). The mechanisms by which miR-125b controls genes involved in fibrogenesis are still to be elucidated. In animal studies, this miRNA was found to be up-regulated in hepatic stellate cells, but not in hepatocytes; in vitro inhibition of miR-125b suppressed the expression of profibrogenic genes in primary hepatic stellate cells cultures (13). An abnormal in vitro expression of miR-125b is also associated with fibrosis in other organs, such as the heart, where this small transcript acts as a potent repressor of apelin and other anti-fibrotic mechanisms (14). Other studies have also reported increased serum levels of miR-125b in chronic HCV infected patients compared to normal controls, together with miR-146a and miR-155 that mediate a pro-inflammatory phenotype of monocytes, triggering TNFα production and maintaining a chronic immune activation, as the characteristics of chronic hepatitis C (15).
Previous reports identified specific miRNAs signatures for the diagnosis of hepatocellular carcinoma and proposed miR-125b expression as a predictor for liver cancer development or survival in HCC (3, 5, 16). We tested patients with chronic hepatitis C, without markers of HCC progression, and identified a direct association between miR-125b up-regulation and advanced stages of liver fibrosis. In liver cancer, miR-125b down-regulation modulates several targets (BCL2, Bcl-W, and interleukin-6 receptor) to interfere with the apoptotic mechanisms and other targets (PIGF, LIN28B) to regulate metastatic invasion and angiogenesis. Intriguingly, miR-125b up-regulation in some tumors indicates the oncogenic potential, while down-regulation in other tumors seems to indicate a tumor suppressive effect (17).
5.1. Conclusions
We suggest that up-regulated miR-125b expression is an indicator of severe liver fibrosis in patients with chronic hepatitis C and might serve as a novel prognostic biomarker, independent of the viral replication. Further studies regarding the longitudinal expression of miR-125b in treated patients are necessary to establish its value for the regression of fibrosis and its potential for therapeutic inhibition.