1. Background
Chronic hepatitis B (CHB) is an important public health threat. It is estimated that 350 - 400 million people are suffering from the disease worldwide. These patients are at a higher risk of liver cirrhosis and hepatocellular carcinoma compared to the general population and about 20% of them are afflicted with cirrhosis (1). In a long-term study on CHB patients, the estimated five-year survival rates were as follows: 97% for patients with chronic persistent hepatitis, 86% for patients with chronic active hepatitis (CAH), and 55% for CAH patients with cirrhosis (2).
Cirrhosis was considered irreversible for a long time, but recent studies proved that the elimination of the underlying cause of liver damage would reverse the fibrosis. The common symptoms of chronic liver disease include weakness, fatigue, and right upper quadrant abdominal pain. It may also present with abnormal laboratory test results (1). Hepatitis B virus (HBV) usually causes chronic infection and leads to cirrhosis, hepatocellular carcinoma, and other severe liver diseases. Diagnostic tests include hepatitis B serology that comprises hepatitis B surface antigen (HBs Ag), hepatitis B surface antibody (HBs Ab), hepatitis B envelope antigen (HBe Ag), hepatitis B envelope antibody (HBe Ab), and hepatitis B Core IgM antibody (HBc Ab IgM). HBV surface antigens are indispensable for HBV virion formation (3). The quantitative assessment of HBV DNA is also necessary. Many studies have demonstrated the benefits of antiviral therapy in patients with CHB. An efficient treatment will suppress the HBV-DNA and reduce aminotransferases level (4). The histological response to treatment is indicated by a decrease in liver inflammation and fibrosis. Various clinical trials have shown that patients with decompensated liver disease can return to the compensated stage using antiviral therapy for hepatitis B (1).
Previous studies in animal models showed that statins are effective in the prevention of liver fibrosis progression, but data from human studies are limited. According to a few available studies, statins may slow down the progression of liver fibrosis in patients with chronic hepatitis C (CHC) (2, 5). In addition, research on a large statistical population reported that adding statins to the antiviral therapy of CHB patients decreases the incidence of cirrhosis and hepatic decompensation significantly (6).
Statins have anti-inflammatory effects. Observations confirm that these drugs reduce the C-reactive protein (CRP) levels in serum (7) and pro-inflammatory cytokines; moreover, they downregulate the activation of T-cells and infiltration of macrophages and neutrophils (8, 9).
Determining the level of parenchymal damage in chronic liver disease is crucial to make the best decision for patient management. For example, a study showed that the level of hepatic and portal veins damage is correlated with the severity of fatty liver disease, but the stage of hepatic parenchymal fibrosis is an independent factor that can be evaluated by FibroScan. They also concluded that the elevation of liver enzymes and serum CRP level correlates with the stage of liver fibrosis (10).
2. Objectives
Given the anti-inflammatory and anti-fibrotic effects of statins and respecting the fact that they are affordable and accessible drugs for patients, the present study aimed to determine the effect of adding atorvastatin to antiviral therapy on hepatic fibrosis or inhibiting the progression of cirrhosis in CHB patients.
3. Methods
This double-blinded clinical trial was approved by the Research Ethics Committee of Qom University of Medical Sciences on January 14, 2017, with the reference code of IR.MUQ.REC.133.1395. It was also registered in the Iranian Registry of Clinical Trials on March 2, 2017, with the reference code of IRCT2017010631252N3.
The statistical population included CHB patients who were eligible for antiviral therapy and attended the gastroenterology and hepatology clinics in Qom, Iran, between 2016 and 2017. According to a study by Trebicka et al. (11) and using the sample size formula and comparing the means of the two groups, 32 patients were assigned to each group. CHB patients with an indication for treatment who had an age of 18 to 75 years, with a serum LDL level of greater than 90 mg/dL, were included consecutively until the calculated sample size reached. The exclusion criteria were: Hepatitis B infection for less than six months; hepatitis B inactive carriers; no signs of liver fibrosis and cirrhosis; decompensated cirrhosis; the presence of other chronic liver diseases such as hemochromatosis and Wilson’s disease or autoimmune hepatitis (12-14) concomitant with hepatocellular carcinoma or any type of malignancy (including digestive and blood), severe and debilitating diseases such as heart, lung, and kidney failure and progressive neurological disorders; coinfection or superinfection with hepatitis A virus (HAV), hepatitis D virus (HDV), hepatitis C virus (HCV), and HIV; any previous use of atorvastatin therapy and lipid-lowering medications; pregnant or lactating females; serum LDL levels of < 90 mg/dL; and elevation of liver enzymes level as twice the normal upper limits within one month of treatment. All patients signed informed consent forms.
Patients’ demographic and laboratory data were collected by a checklist. The Child-Pugh Score was measured using the data from the checklist (Tables 1 and 2). Liver stiffness in all the patients was assessed by FibroScan device (FibroScan® 402, Echosens, Paris, France). Patients were grouped as intervention and control by simple random sampling. The intervention group received tenofovir (300 mg) and atorvastatin (20 mg) and the control group received tenofovir (300 mg) and placebo on a daily basis. The medications and placebo were purchased from the Abidi Pharmaceutical Company, Tehran, Iran. Placebo was made of starch material in the shape and size of Atrovastatin pills.
| Clinical and Lab Criteria | Points* | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Encephalopathy | None | Mild to moderate (grade 1 or 2) | Severe (grade 3 or 4) |
| Ascites | Mild to moderate (diuretic responsive) | Severe (diuretic responsive) | |
| Bilirubin, mg/dL | < 2 | 2 - 3 | > 3 |
| Albumin, g/dL | > 3.5 | 2.8 - 3.5 | < 2.8 |
| Prothrombin time | |||
| Second prolonged | < 4 | 4 - 6 | > 6 |
| International normalized ratio | < 1.7 | 1.7 - 2.3 | > 2.3 |
aChild-Turcotte-Pugh class obtained by adding score for each parameter (total points): Class A = 5 to 6 points (least severe liver disease), class B = 7 to 9 points (moderately severe liver disease), class C = 10 to 15 points (most severe liver disease).
| Score | Details |
|---|---|
| Activity score (A) | |
| A0 | No activity |
| A1 | Mild activity |
| A2 | Moderate activity |
| A3 | Severe activity |
| Fibrosis score (F) | |
| F0 | No fibrosis |
| F1 | Portal fibrosis without septa |
| F2 | Portal fibrosis with few septa |
| F3 | Numerous septa without cirrhosis |
| F4 | Cirrhosis |
In order to maintain the study blind, the drugs were coded and administered to the patients by a person, other than the attending physician, who was unaware of the drugs. One month after the treatment, liver enzymes were checked and in case of the elevation of enzymes’ level more than twice the normal upper limits, the patients were excluded from the study. Liver enzymes were checked monthly. In the sixth month, the liver function, liver enzyme level, and liver stiffness were re-measured.
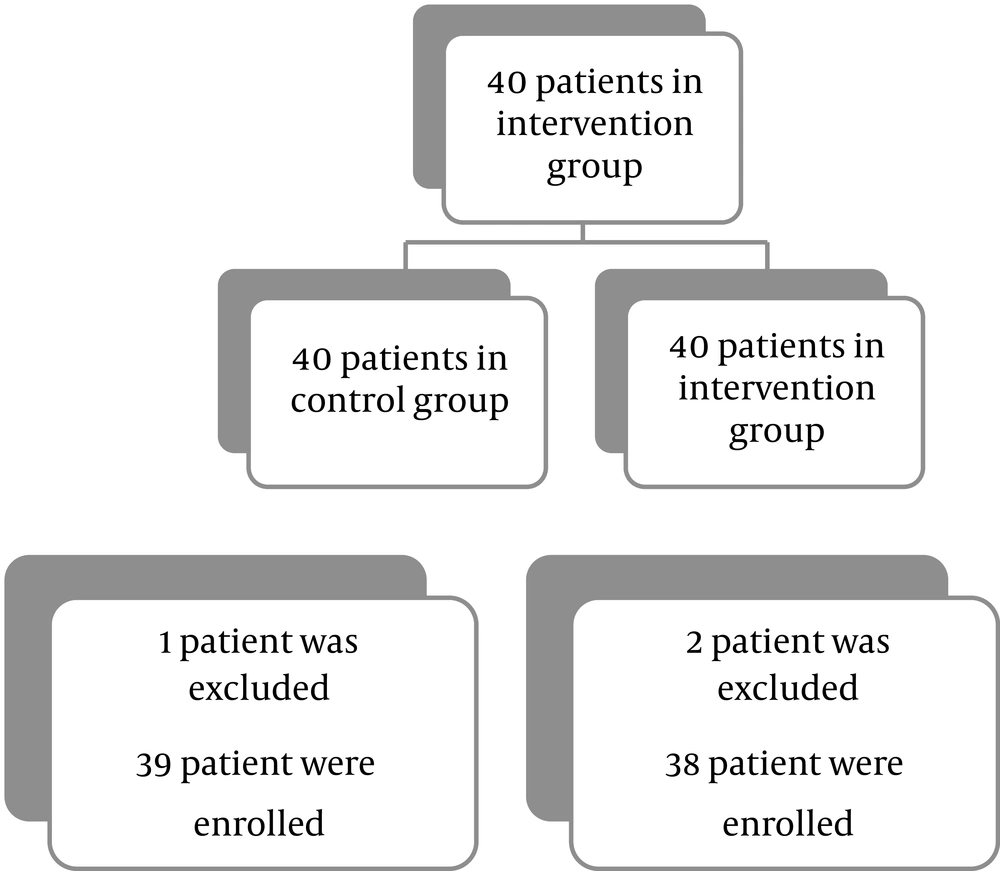
Among 40 patients in each group, one from the control group and two from the intervention group were excluded because they failed to take part in the follow-up examination. Finally, 39 patients in the control group and 38 patients in the intervention group completed the study (Figure 1).
Data were analyzed with statistical package for social sciences (SPSS) version 24 using descriptive statistics including frequency, mean, and standard deviation to interpret the findings. Statistical tests applied in the study included the chi-square test, the independent t-test, and the paired t-test. P values of < 0.05 were considered statistically significant.
4. Results
Among 39 patients in the control group, 27 were male and 12 were female. From among 38 patients in the intervention group, 21 were male and 17 were female. The distribution of gender (P = 0.72), age (P = 0.59), and BMI (P = 0.72) did not show any statistically significant difference between the two groups. The mean levels of aspartate aminotransferase (AST) (P = 0.41), alanine aminotransferase (ALT) (P = 0.17), and liver fibrosis according to the FibroScan findings (P = 0.72) before the treatment were statistically similar between the two groups, which indicates the appropriate randomization of the patients. Table 3 summarizes patients’ demographics, laboratory findings, and transient elastography results of the two groups. Changes in the ALT level were not significant in both groups (P = 0.266). AST level changes were non-significant in the control group (P = 0.893), but significant in the intervention group (P = 0.018). Changes in the transient elastography results were significant only in the intervention group (P = 0.001). This finding indicates the efficacy of statins in improving liver fibrosis. Table 4 compares the means of the live enzyme levels and the transient elastography results before and after the treatment in both groups. Table 5 shows the frequency and percentage of the patients according to the METAVIR scoring system after the intervention in both groups. Table 6 shows changes in the stage of liver fibrosis following the treatment in both groups. There was no statistically significant difference between the two groups after the intervention respecting the frequency percentage of transient elastography (P = 0.064). Pre-intervention and post-intervention transient elastography were significantly associated with tenofovir and placebo in the control group (P = 0.001). This reveals the relative effect of tenofovir on improving the liver histology in CHB patients. Moreover, in the intervention group, this association was significant before and after the intervention (P = 0.001). There were no significant changes in the Child-Pugh score in the control group after the treatment (P = 0.66); however, the changes were significant in the intervention group (P < 0.001).
| Variables | Control Group (N = 39)a | Intervention Group (N = 38)a | Mean Difference | T | Degree of Freedom | P Value |
|---|---|---|---|---|---|---|
| Age | 53.47 ± 68.13 | 24.49 ± 69.14 | -711.1 | -0.525 | 74 | 0.601 |
| BMI | 13.24 ± 94.4 | 86.24 ± 56.5 | -723.0 | -0.541 | 74 | 0.590 |
| First AST | 44.33 ± 13.23 | 86.29 ± 57.13 | 581.3 | 0.826 | 75 | 0.412 |
| First ALT | 51.39 ± 94.30 | 71.31 ± 39.17 | 802.7 | 1.359 | 75 | 0.178 |
| Second AST | 54.32 ± 42.15 | 95.31 ± 73.10 | 591.0 | 0.195 | 75 | 0.846 |
| Second ALT | 90.38 ± 23.18 | 45.37 ± 03.18 | 450.1 | 0.351 | 75 | 0.727 |
| First Transient Elastography test | 63.10 ± 63.12 | 52.11 ± 52.8 | -887.0 | -0.359 | 74 | 0.721 |
| Second Transient Elastography test | 64.10 ± 05.13 | 09.9 ± 15.6 | 552.1 | 0.663 | 74 | 0.040 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase, BMI, body mass index.
aValues are expressed as mean ± SD.
| Variables | Control Group (N = 39) | Intervention Group (N = 38) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Mean Difference | T | P Value | Pre-Intervention | Post-Intervention | Mean Difference | T | P Value | |
| AST | 44.33 (13.23) | 54.32 (42.15) | 89.0 | 293.0 | 771.0 | 86.29 (57.13) | 95.31 (73.10) | -092.2 | -131.1 | 0.266 |
| ALT | 51.39 (94.30) | 90.38 (23.18) | 615.0 | 136.0 | 893.0 | 71.31 (39.17) | 45.37 (03.18) | -737.5 | -476.2 | 0.018 |
| Transient Elastography test | 63.10 (63.12) | 64.10 (05.13) | -053.0 | 442.0- | 661.0 | 52.11 (52.8) | 09.9 (15.6) | 500.0 | 346.4 | 0.001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
| Group | Last Transient Elastography Test | Chi-Square | Degree of Freedom | P Value | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| Control | 17 (7.44) | 15 (5.39) | 4 (5.10) | 2 (3.5) | 668.1 | 3 | 0.644 |
| Intervention | 20 (6.52) | 11 (9.28) | 3 (9.7) | 4 (5.10) | |||
aValues are expressed as No. (%).
| First Transient Elastography test | Last Transient Elastography Test | Chi-Square | Degree of Freedom | P Value | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| Control | 015.59 | 9 | 0.001 | ||||
| 1 | 15 (8.93) | 1 (3.6) | - | - | |||
| 2 | 2 (5.12) | 12 (75) | 2 (5.12) | - | |||
| 3 | - | 1 (3.33) | 2 (7.66) | - | |||
| 4 | - | 1 (3.33) | - | 2 (7.66) | |||
| Intervention | 173.42 | 9 | 0.001 | ||||
| 1 | 16 (1.94) | 1 (9.5) | - | - | |||
| 2 | 4 (50) | 4 (50) | - | - | |||
| 3 | - | 4 (80) | 1 (20) | - | |||
| 4 | - | 2 (25) | 1 (25) | 4 (50) | |||
aValues are expressed as No. (%).
5. Discussion
According to the results of the present study, there were no significant changes in the AST level in the two groups. The changes in the ALT level before and after treatment were significant in the intervention group, but compared to the control group, no significant changes were detected. According to the findings of the present study and some previous studies, patients consuming these drugs are not at a higher risk of liver enzyme disturbance (13, 14).
Assessing the liver fibrosis after treatment represented a significant reduction in the stage of fibrosis, which indicates the efficacy of antiviral treatment for improving liver histology. These findings are consistent with the results of studies by Hou et al. (15) and Li et al. (16).
Statins have anti-inflammatory effects and can reduce the serum levels of CRP and pro-inflammatory cytokines (7). Since the inflammatory cascade, which starts with the signals of liver stellate cells following hepatocytes damage, may lead to hepatitis and hepatic fibrosis, the anti-inflammatory and anti-fibrotic effects of statins may control the liver damage (8, 17). Some studies have shown that statins might offer clinical benefits in viral hepatitis, progression of cirrhosis, and hepatocellular carcinoma; moreover, statins were shown to ameliorate liver histological (in both imaging and biopsy studies) and functional alterations in patients with non-alcoholic fatty liver disease or non-alcoholic steatohepatitis (18), although the present study did not indicate histological amelioration.
Simon et al. in 2015 evaluated the effectiveness of statins in reducing the hepatic fibrosis progression in patients with CHC. In this study, subjects with CHC and advanced fibrosis underwent serial liver biopsies over 3.5 years. The study results demonstrated that fibrosis progression occurred in 10% of statin users and 29% of non-users. It could be concluded that using statins is associated with a reduced risk of hepatic fibrosis progression in advanced CHC (19). This finding is in line with the results of the present study.
Chen et al. explored the effects of statin and metformin on the incidence of liver or non-liver cancers in patients suffering from chronic infection with HBV during 2000 - 2008. The study revealed that either statin or metformin reduced the incidence of cancer. It also demonstrated that consuming both statin and metformin had a synergistic effect and reduced the incidence of many cancers (20). In the current study, complementary therapy was found to be more effective than antiviral therapy alone in improving liver fibrosis; however, the present study did not aim to determine the risk of liver cancer.
Kamal et al. in 2017 conducted a systematic review in the United States of America. It indicated that statins could retard the progression of fibrosis, prevent hepatic decompensation in cirrhosis, and decrease mortality in patients with chronic liver disease (21). Likewise, the findings of the present study showed that atorvastatin therapy reduced liver fibrosis significantly.
A study carried out by Huang et al. in Taiwan between 2007 and 2009 demonstrated that CHB patients who received statin therapy in combination with antiviral therapy had a significantly lower cumulative incidence of cirrhosis and decompensated cirrhosis compared to patients who did not take statins (6). These are consistent with the results of the present study that indicated adding statins to antiviral therapy would improve liver fibrosis. A study showed that statins might reduce liver-related adverse outcomes in chronic liver disease. Kamal et al. in a recent meta-analysis found that statin use was associated with a reduced risk of fibrosis progression, decompensated cirrhosis, and mortality (22). Similarly, our study showed that there are beneficial liver effects for statins. More-robust studies including randomized trials and research on its safety in patients with advanced liver disease are needed before statins can be routinely recommended for the prevention of liver-related morbidity and mortality.
5.1. Conclusions
By setting the results of the present study against the findings of other studies, it is concluded that using atorvastatin therapy in combination with standard antiviral treatment has a positive impact on liver fibrosis and dysfunction in CHB patients. Furthermore, through its anti-inflammatory and anti-fibrotic effects, atorvastatin leads to the regression of fibrosis in the early stages of liver fibrosis, thereby improving liver function. In addition, these drugs are affordable and accessible to patients and all health plans cover them. Hence, it is suggested that atorvastatin be used as complementary therapy.