1. Background
Hepatitis A virus (HAV) belongs to the picornavirus family and causes liver dysfunction in a considerable ratio of populations (1). HAV infection is transmitted through the oral-fecal route. HAV can lead to widespread epidemics and mortality in adults, especially in societies with poor health conditions; nevertheless, the mortality and morbidity rates are low in pediatrics (2).
This disease is usually self-limiting. A small ratio of patients with HAV shows the recurrence of hepatitis from weeks to months after initial improvement. The recurrence is usually associated with increased aminotransferases and jaundice. Uncommonly, HAV may present as cholesteric and fulminant hepatitis that is characterized by prolonged cholestatic jaundice and itching (3, 4).
The diagnosis of HAV is made by serological tests and biochemical evaluation of liver function. The acute HAV infection is characterized by increased levels of AST and ALT due to prominent damage to hepatocytes. The maximum levels of these enzymes usually reach during the icteric phase of the disease while the enzymes gradually decrease during the recovery phase (5, 6).
Ursodeoxycholic acid (UDCA) is a secondary dihydrofolic dihydroxy bile acid with hydrophilic properties (7). UDCA has been used to treat many diseases including biliary cirrhosis, primary sclerosing cholangitis, cystic fibrosis, and non-alcoholic fatty liver disease (8). UDCA has shown to reduce serum bilirubin, ALT, AST, ALP, and gamma-glutamyl transpeptidase (GGT), especially in cholestatic patients such as those with primary biliary cirrhosis (PBC) and scleral cholangitis (9). This drug also improves cholesteric and cytologic parameters in patients with chronic liver diseases such as chronic hepatitis and cystic fibrosis (10). UDCA has also been used as a complementary drug in adult patients with acute and chronic viral hepatitis infections (11, 12).
2. Objectives
The mechanism of UDCA action is unclear. It has been proposed that UDCA has cytoprotective and anti-apoptosis effects (13). Studies have shown that this drug increases the conversion of cholesterol into bile acids (13). There is no report of the effects of UDCA on the levels of hepatic liver enzymes in children with acute HAV infection. Therefore, this study aimed to monitor the fluctuations of liver enzymes in children with acute HAV infection treated with either UDCA or placebo.
3. Methods
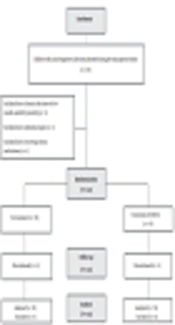
This single-blinded clinical trial was conducted on children diagnosed with acute HAV infection referring to Imam Khomeini hospital of Zabol, Iran, in 2017. This study was approved by the Ethics Committee of Zabol University of Medical Sciences (ethical code: Zbmu.1.REC.1396.65) and registered in the Iranian Registry of Clinical Trials (IRCT20171113037429N1). The parents were asked to read and sign an informed consent form after finding their children were eligible for inclusion in the study. The study was carried out according to the CONSORT guideline for performing clinical trials (Figure 1).
3.1. Sample Size
According to the endpoint (i.e. improvement in liver enzymes), the sample size was decided based on a previous clinical trial with a similar design performed on patients with viral hepatitis (14).
3.2. Inclusion Criteria
We included children who had positive anti-HAV IgM result along with either coagulopathy (international normalized ratio > 1.5), encephalopathy (as diagnosed by a neurologist according to the presence of neurological and psychological dysfunctions such as altered attitude, impaired speech abilities, sleep disorders, and locomotor dysfunction accompanied by abnormal electroencephalogram results), or intolerance to oral feeding (i.e. intractable vomiting).
3.3. Exclusion Criteria
We excluded children with a history of HAV positivity for up to six months, abdominal pain, color change in urine or stool, or those under medications for any other reason. Serological tests of anti-HCV and anti-HBsAg were used to exclude HBV/HCV-infected children.
3.4. Treatments
The eligible participants were randomly divided into intervention and control groups. A list of random numbers was created using an online randomizer tool (https://www.randomizer.org). The intervention group received oral UDCA (exquisite, 10 - 15 mg/kg body weight, 2 - 3 times daily in association with their meals). The control group did not receive any medication. The patients were not aware of the treatment they received (i.e. either UDCA or placebo).
3.5. Liver Enzyme Measurements
ALT and AST enzymes were measured before the intervention, as well as two weeks, four weeks, two months, three months, four months, and six months after the intervention. For measurements, 5-mL blood samples were collected into tubes containing no additives by a trained laboratory technician each time. The samples were then transferred to the hospital laboratory and sera were separated by centrifugation. Liver enzymes along with other biochemical parameters were measured by specific ELISA kits manufactured by Pars Azmoun company (Iran). The sensitivities of AST and ALT kits were 2 IU/L and 4 IU/L, respectively. The mean intra-assay and inter-assay precisions (low, normal, and high values) were 2.36% and 2.15% for the AST kit and 3.28% and 1.86% for the ALT kit, respectively.
3.6. Statistical Analysis
SPSS version 19 was used for statistical analysis and p values of less than 0.05 were considered statistically significant. The normal distribution of the data was checked by the Kolmogorov-Smirnov test. The mean liver enzymes before and after the study were compared between the two groups using repeated measures ANOVA test.
4. Results
In this study, 152 children with acute HAV infection were enrolled. All the patients were icteric at the time of admission. They were randomly allocated to either the UDCA group or the control group (n = 76 per group). There were 81 (53.3%) boys among the patients. The mean age of the participants was 83.28 ± 39.7 months. The lowest and the highest ages were six months and 18 years, respectively (Table 1).
| Features | Control, N = 76 | UDCA, N = 76 | P Value |
|---|---|---|---|
| Gender, No. (%) | 0.6 | ||
| Male | 39 (51.3) | 42 (55.3) | |
| Female | 37 (48.7) | 34 (44.7) | |
| Age, y | 6.4 ± 2.5 | 7.4 ± 3.8 | 0.07 |
| WBC, ×103/µL | 7517.3 ± 2524.4 | 7871.7 ± 4277.4 | 0.53 |
| Haemoglobin, g/dL | 11.8 ± 1.5 | 12.3 ± 1.6 | 0.05 |
| Platelet, ×109/µL | 351.5 ± 120.8 | 352.9 ± 154.7 | 0.9 |
| Albumin, g/dL | 4.39 ± 0.8 | 4.5 ± 1.12 | 0.4 |
| Total bilirubin, mg/dL | 4.91 ± 5.24 | 4.95 ± 3.21 | 0.9 |
| Direct bilirubin, mg/dl | 2.59 ± 3.55 | 2.68 ± 2.07 | 0.8 |
| Total protein, g/dL | 6.98 ± 0.84 | 6.95 ± 1.18 | 0.8 |
Basic Characteristics of Children Enrolled in the Study
In the UDCA group, the ALT level declined from 1296.7 ± 1236 IU/L at the beginning of the study to 15.4 ± 3 IU/L after six months. In the control group, the ALT level changed from 1062.3 ± 959 IU/L at the beginning of the study to 16.4 ±5 IU/L after six months (Table 2).
| Timepoints | ALT Levels, IU/mL | P Valueb | Normal ALT Levels | P Value | ||
|---|---|---|---|---|---|---|
| Control, N = 76 | UDCA, N = 76 | Control, N = 76 | UDCA, N = 76 | |||
| Before treatment | 1062.3 ± 959 | 1296.7 ± 1236 | 0.1 | 2 (2.6) | 0 (0) | 0.1 |
| Two weeks post-treatment | 313.6 ± 797 | 206.7 ± 308 | 0.2 | 15 (20) | 9 (11.8) | 0.1 |
| One month post-treatment | 77.6 ± 110 | 53.2 ± 37 | 0.06 | 36 (47.4) | 36 (47.4) | 0.9 |
| Two months post-treatment | 37.4 ± 30 | 32.9 ± 20 | 0.2 | 51 (68) | 61 (80.3) | 0.06 |
| Three months post-treatment | 26.4 ± 11 | 23.5 ± 11 | 0.1 | 68 (90.7) | 70 (92.1) | 0.7 |
| Four months post-treatment | 20.5 ± 7 | 18.6 ± 5 | 0.05 | 73 (97.3) | 75 (98.7) | 0.5 |
| Six months post-treatment | 16.4 ± 5 | 15.4 ± 3 | 0.1 | 76 (100) | 76 (100) | - |
| P valuec | < 0.0001 | < 0.0001 | ||||
Mean ALT Changes Over Six Months in Children Receiving UDCA or No Treatmenta
Likewise, in the UDCA group, the mean AST level declined from 983.8 ± 1036 IU/L at baseline to 19.4 ± 5 IU/L at the end of six months. This is while AST reduced from 981.8 ± 1177 IU/L at the beginning of the study to 22.8 ± 7 IU/L after six months in the control group (Table 3). No adverse effects were observed in patients receiving UDCA.
| Timepoints | AST Levels, IU/mL | P Valueb | Normal AST Level | P Value | ||
|---|---|---|---|---|---|---|
| Control, N = 76 | UDCA, N = 76 | Control, N = 76 | UDCA, N = 76 | |||
| Before treatment | 981.8 ± 1177 | 983.8 ± 1036 | 0.9 | 3 (3.9) | 0 (0) | 0.08 |
| Two weeks post-treatment | 245.8 ± 561 | 135 ± 191 | 0.1 | 8 (10.7) | 11 (14.5) | 0.4 |
| One month post-treatment | 68 ± 47 | 52.7 ± 29 | 0.01 | 20 (26.7) | 34 (44.7) | 0.02 |
| Two months post-treatment | 45.4 ± 21 | 32.2 ± 14 | 0.001 | 31 (41.3) | 51 (67.1) | 0.001 |
| Three months post-treatment | 33.9 ± 11 | 27.9 ± 10 | 0.001 | 56 (74.7) | 66 (86.8) | 0.04c |
| Four months post-treatment | 28.7 ± 11 | 23.7 ± 8 | 0.002 | 69 (92) | 73 (96.1) | 0.2 |
| Six months post-treatment | 22.8 ± 7 | 19.4 ± 5 | 0.001 | 74 (98.7) | 76 (100) | 0.3 |
| P valued | < 0.0001 | < 0.0001 | ||||
Mean AST Changes Over Six Months in Children Receiving UDCA or No Treatmenta
5. Discussion
Treatment strategy in patients with acute viral hepatitis, including HAV infection, is based on supportive interventions, as well as anti-viral treatments in symptomatic cases. Potential beneficiary effects of UDCA are unclear in the clinical course of childhood acute HAV. In the present study, we demonstrated that UDCA had some beneficial effects on children with acute HAV infection. Although there was no significant difference in the rate of AST or ALT reductions at the end of six months of therapy, a higher ratio of patients in the UDCA group (61, 80.3%) than in the control group (51, 68%) had normal ALT levels at the end of two months (P = 0.06). Accordingly, the ratio of patients with normal AST level was significantly higher in the UDCA group than in the control group at the end of the first month (44.7% vs. 26.7%, respectively; P = 0.02), two months (67.1% vs. 41.3%, respectively; P = 0.001), and three months (86.8% vs. 74.7%, respectively; P = 0.04) after therapy initiation.
UDCA has been described as an effective agent in improving some disease markers in adult patients with acute or chronic viral hepatitis. In a study by Fabris et al. on 79 adult patients with acute viral hepatitis including 15 patients with acute HAV infection, hepatic aminotransferases levels showed no significant differences between patients who received UDCA for three weeks and non-treated patients (15). In a meta-analysis by Chen et al. it was shown that UDCA was effective to alleviate the levels of HBsAg, HBV DNA, and hepatic aminotransferases in patients with HBV infection (16). In addition, patients with HCV infection treated with UDCA had significantly lower hepatic enzymes levels than those receiving placebo (16). Moreover, treatment with UDCA for one year reduced hepatic enzymes levels and accelerated viral clearance in patients affected with acute viral hepatitis (17). In patients with HCV infection, incorporating UDCA into the therapeutic protocol significantly improved ALT levels in patients who either could not tolerate (18) or did not respond to (19, 20) interferon-based therapies. Other studies also showed that UDCA dose-dependently reduced AST, ALT, and GGT levels in patients with chronic HCV infection (19, 21, 22). In the comparison between the two groups of HCV-infected patients who were treated with either alpha-IFN or alpha-IFN+UDCA, the latter group had significantly higher reductions in ALT levels at the end of six months following therapy (20). According to another study by Attily et al. UDCA administration reduced transaminases and GGT peptidases in patients with chronic active hepatitis (23). In another study conducted by Leuschner et al. UDCA reduced serum levels of ALT and AST in patients with biliary cirrhosis and chronic hepatitis (24). Similar results were also observed in studies carried out by Bellentani et al. (25) and Rolandi et al. (26) who showed that UDCA significantly affected hepatic liver enzymes in patients with chronic active hepatitis.
Contrary to previous studies, Galsky et al. reported no significant differences in liver enzymes comparing HBV-infected patients treated with UDCA and with placebo after 12 months of therapy (17). Nevertheless, we here observed that UDCA accelerated the reduction in hepatic enzymes in children with acute HAV infection. In this regard, the ratio of patients with normal AST levels was significantly higher in the UDCA group than in the control group one month (n = 34, 44.7% vs. n = 20, 26.7%, respectively; P = 0.02), two months (n = 51, 67.1% vs. n = 31, 41.3%, respectively; P = 0.001), and three months (n = 66, 86.8% and n = 56, 74.7%, respectively; P = 0.04) post-therapy. Likewise, the ratio of patients with normal ALT levels was higher in the UDCA group (61, 80.3%) than in the control group (51, 68%) two months post-therapy (P = 0.06). Six months post-therapy, however, all the patients reached normal AST and ALT levels in both groups.
Therapeutic response to UDCA can be influenced by the clinical characteristics of patients with acute or chronic hepatitis. In this regard, superior responses to UDCA treatment may be achieved in patients with lower initial levels of hepatic aminotransferases and higher initial levels of GGT and those with liver cirrhosis (27). In additions, genetic factors and other inter-individual variables may also influence the therapeutic outcomes, which need to be assessed in future population-based studies.
Detailed downstream mechanisms involved in therapeutic actions of UDCA are yet to be elucidated. As noted, UDCA is a secondary dihydrofolicdihydroxyl bile acid with hydrophilic properties (7). It is assumed that one of the main mechanisms of UDCA protective actions in cholestatic liver disease is to replace hepatotoxic bile acids within hepatocytes (28). In experimental models of liver cholestatic diseases, the accumulation of hydrophobic bile acids such as chenodeoxycholic and deoxycholic acids in hepatocytes caused multiple cellular damages including increased fluidity and permeability of hepatocytes membranes, cell death (apoptosis), and necrosis (15). The extent and the duration of liver exposure to these hepatotoxic bile acids determine the extent of damage to hepatocytes, and transient accumulation of these toxicants can reversibly increase transaminases (29). The substitution of these bile acids with more hydrophilic components (i.e. UDCA) can stabilize hepatocytes membranes and protect these cells from apoptosis. UDCA also has immunomodulatory effects by inhibiting IFN-γ production by intrahepatic lymphocytes (30). Furthermore, UDCA can also induce the release of cytochrome c from mitochondria and suppress apoptosis in hepatocytes (15). In addition, anti-apoptosis, anti-inflammatory, and antioxidant properties of UDCA have been shown in neural cells exposed to high levels of bilirubin (28, 31). Other potential molecular events involved in UDCA protective actions against hepatic damage are yet to be elucidated.
5.1. Limitations
Although AST and ALT can reliably reflect the liver health, histological examination is the gold standard for assessing liver functional status. In uncomplicated HAV infection, however, histological examinations are rarely indicated. The histological effects of UDCA may help more reliably conclude on the applicability of UDCA in clinical settings.
5.2. Conclusions
Our findings showed that UDCA administration in children with acute HAV infection accelerated the normalization of liver enzymes. However, there were no significant differences in the ratios of AST and ALT normalization between children receiving UDCA and non-treated children after six months of therapy.