1. Background
Chronic hepatitis C virus (HCV) infection is a significant public health problem, leading to the development of cirrhosis in 20 % of cases and hepatocellular carcinoma in 1% - 5% of cases (1, 2). In European countries, HCV-related cirrhosis is the most common indication for liver transplantation (3). The recurrence of HCV formerly was one of the main causes of graft loss. Furthermore, immunosuppressive therapy may promote the development of liver fibrosis (4). Direct-acting antivirals (DDAs) changed this situation. Transplanted patients show high sustained viral response (SVR) rates and tolerability (5, 6) but data concerning interactions between DAAs and immunosuppressants are scarce (7).
2. Objectives
To understand the relationship between DAAs and immunosuppressants, we studied the epidemiology of patients with HCV recurrence after liver transplantation, treated with different DAAs combinations, with or without ribavirin. Moreover, we evaluated the SVR rates and tolerability, as well as the interactions between immunosuppression and antiviral therapy.
3. Methods
3.1. Study Setting, Design, and Patient Selection
This monocentric retrospective observational study included all transplanted patients (≥ 18 years of age) treated with DAAs between November 2014 and September 2017 at “Ospedali Riuniti”, Ancona, due to the recurrence of HCV infection. Demographic, clinical, and virological characteristics and antiviral treatment were compared between SVR and non-SVR patients. Clinical data were collected at baseline, 4 and 12 weeks after the beginning of treatment, at the end of therapy, and 12 and 24 weeks after treatment. Liver fibrosis was assessed by TE and classified as described by Castera et al. (8). The aspartate aminotransferase to platelet ratio index (APRI) score and the Fibrosis 4 score (FIB-4) were calculated, both at the beginning and 12 weeks after the end of antiviral therapy. The SVR was defined as non-detectable HCV RNA after 12 weeks of treatment completion. The HCV RNA levels were tested by the Roche test (sensitivity of 12 IU/mL). The study was performed following the ethical standards of the 1964 Declaration of Helsinki and later amendments. The “Ospedali Riuniti” Ethics Committee granted retrospective access to data without informed consent.
3.2. Statistical Analysis
The data were analyzed by SPSS 20.0 software. Categorical variables are expressed as absolute numbersand relative frequencies. Continuous variables are expressed as means, median, and interquartile range. Categorical variables were compared with the χ2 or Fisher’s exact test while continuous variables were evaluated by Student’s t test or the Mann-Whitney U test. The P values of ≤ 0.05 were considered statistically significant.
4. Results and Discussion
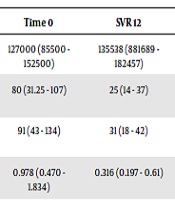
In total, 55 patients with post-transplant recurrence of HCV infection were evaluated (Table 1 and Appendix 1 in Supplementary File). The mean age of the patients was 56.4, and 78% (n = 43) of them were men. Moreover, 25 patients presented hepatocellular carcinoma before liver transplant and 3 patients were HIV co-infected. The genotype data were available for 48 patients, with genotype 1a being the most frequent one. The fibrosis stage was F0 - F1 in 11 patients (20%), F2 in 15 patients (27%), F3 in 6 patients (11%), and F4 in 8 patients (15%). In addition, 22 patients received treatment within 12 months of transplantation. Moreover, 15 patients were classified as relapsers or non-responders to pegylated interferon combined with ribavirin. Thirty patients (54%) received sofosbuvir + daclatasvir (SOF + DAC), 10 (18%) sofosbuvir + ribavirin (SOF + RBV), 7 (13%) sofosbuvir + simeprevir (SOF + SIM), 7 (12%) sofosbuvir + ledipasvir (SOF + LED), and 1 (2%) sofosbuvir + velpatasvir (SOF + VELP). The treatment was undertaken for 12 weeks in 21 cases and for 24 weeks in 33 cases. Ribavirin was attributed to 80% of the patients. The SVR 12 was achieved in 89% of the patients, including 90% with SOF + DAC, 80% with SOF + RBV, 85,7% with SOF + SIM, and 100% with SOF + LED and SOF + VELP. The SVR was achieved in all patients with fibrosis stage F0 - F1 and F3, 87% of the recipients with stage F2, and 50% of the cirrhotic patients. Notably, 66% of the patients that did not achieve SVR showed an F4 fibrosis stage, which was statistically significantly different compared to the SVR group (P = 0.002). One patient relapsed after 24 weeks. Among the 6 relapsed patients, 4 had genotype 1a and 2 had genotype 3. In addition, 5 of them were retreated: 3 with SOF + DAC, 1 with SOF + SIM, and 1 with SOF + VELP. Only the latter patient relapsed again, 12 weeks after the end of therapy. These data are slightly inferior to those obtained in different studies (9, 10). Biochemical parameters showed an improvement in 12 weeks after therapy in patients with SVR even though only AST and ALT decreases were statistically significant (Table 2). The median value of APRI and FIB-4 were 0.978 and 3.094 at the start of therapy and 0.316 and 1.52 at SVR 12, respectively (P = 0.0125 and P < 0.00001). There were 15 patients developing anemia: one of them discontinued treatment due to severe anemia but the others reduced ribavirin. One patient had a heart attack, one had a surrenal metastasis and another one died of sepsis three months after the end of therapy. Concerning immunosuppressants, the most frequent immunosuppressive drug used was tacrolimus (85%), followed by everolimus (68%) used in combination in 58% of the patients. Tacrolimus dosage was modified in 52% (26/55) of the patients (including 18 patients with increased dosage and 8 patients with decreased dosage) during the first three months of therapy with DAAs, divided as follows: 37.5% (3/8) of the patients treated with SOF + SIM, 55.6% (15/27) with SOF + DAC, 60% (3/5) with SOF + LED, 55.6% (5/9) with SOF + RIBA, and none with SOF + VELP. In the last three months of therapy, the dosage of tacrolimus was increased in 8 out of 34 patients who had completed 6 months of DAAs therapy. Concerning everolimus, in the first three months, the dosage was modified in 28.9% (13/41) of the patients (including 8 patients with increased dosage was raised and 5 patients with decreased dosage), divided as follows: 42.9% (4/7) of the patients treated with SOF + SIM, 26.1% (6/23) with SOF + DAC, 40% (2/5) with SOF + LED, 22.2% (2/9) with SOF + RIBA, and none with SOF + VELP. In the other three months of DAAS therapy, the everolimus dosage was increased in 19.4% (6/31) of the patients. No significate differences were found between patients with and without a change in the immunosuppressant dosage. However, we noted a trend of change in immunosuppressant dosages during the DAAs therapy, in particular with tacrolimus. Drug-drug interaction between immunosuppressants and first-generation DAAs is noted, but data about the new generation of DAAs are insufficient (11).
| Patients Characteristics | All (55) | SVR (49) | Non-SVR (6) | P Value |
|---|---|---|---|---|
| Mean age, y | 56.4 | 57 | 54 | 0.55 |
| Male, No. (%) | 43 (78) | 39 (79) | 4 (67) | 0.30 |
| Mean BMI | 23.6 | 24.2 | 23 | 0.50 |
| ALT (UI/L), baseline | 92 (52 - 134) | 91 (43 - 134) | 89 (24 - 164) | 0.92 |
| AST (UI/L), baseline | 100 (47 - 154) | 80 (31.25 - 107) | 100 (22 - 200) | 0.81 |
| Platelets/mmc, baseline | 98048 (51277 - 145538) | 127000 (85500 - 152500) | 91000 (52000 - 159250) | 0.70 |
| HCV RNA (UI/mL), mean (95% CI) | 6291063.3 (616376.7 - 6777154.2) | 6734631.6 (668552.5 - 9608330) | 2121520.8 (617634 - 3213866) | 0.41 |
| Genotype, No. (%) | ||||
| 1a | 20 (36) | 16 (33) | 4 (67) | 0.23 |
| 1b | 11 (20) | 11 (22) | 0 | 1 |
| 2 | 0 | 0 | 0 | 1 |
| 3 | 15 (27) | 13 (27) | 2 (33) | 0.65 |
| 4 | 2 (4) | 2 (4) | 0 | 1 |
| Unknown | 7 (13) | 7 (14) | 0 | 1 |
| Fibrosis stage, No. (%) | ||||
| F0 - F1 | 11 (20) | 11 (22) | 0 | 0.33 |
| F2 | 15 (27) | 13 (27) | 2 (33) | 0.65 |
| F3 | 6 (11) | 6 (12) | 0 | 1 |
| F4 | 8 (15) | 4 (8) | 4 (66) | 0.002 |
| Unknow | 15 (27) | 15 (31) | 0 | 1 |
| Immunosoppression No. (%) | ||||
| Tacrolimus | 51 | 45 (92) | 6 (100) | 1 |
| Ciklosporin | 1 | 1 (2) | 0 | 1 |
| Everolimus | 41 | 35 (71) | 6 (100) | 0.32 |
| Mycophenolate mofetil | 5 | 5 (10) | 0 | 1 |
| DAAs, No. (%) | ||||
| SOF + SMV | 7 (13) | 6 (12) | 1 (17) | 0.57 |
| SOF + RIBA | 10 (18) | 8 (16) | 2 (33) | 0.29 |
| SOF + DAC | 30 (54) | 27 (55) | 3 (50) | 1 |
| SOF + LDV | 7 (13) | 7 (14) | 0 | 1 |
| SOF + VEL | 1 (2) | 1 (2) | 0 | 1 |
Baseline Characteristics of 55 Patients with Post-Transplant Recurrence of Hepatitis Ca
| Time 0 | SVR 12 | P Value | |
|---|---|---|---|
| Platelets/mmc, mean (range) | 127000 (85500 - 152500) | 135538 (881689 - 182457) | 0.332 |
| AST, UI/L, mean (range) | 80 (31.25 - 107) | 25 (14 - 37) | < 0.00001 |
| ALT, UI/L, mean (range) | 91 (43 - 134) | 31 (18 - 42) | < 0.00001 |
| APRI, median (range) | 0.978 (0.470 - 1.834) | 0.316 (0.197 - 0.61) | 0.012 |
| FIB-4, median (range) | 3.094 (1.587 - 4.551) | 1.52 (1.05 - 2.72) | < 0.00001 |
Biochemical Parameters, APRI, and FIB4 at the Start of Therapy and at SVR 12a
One study reported the variability of tacrolimus plasma concentration during HCV therapy, probably due to liver function improvement and increment of tacrolimus metabolism (12).
Our study has some limitations. It is a monocentric retrospective study with small sample size. Moreover, there was no information about treatment with new antivirals. Nevertheless, our study confirmed the high efficacy and tolerability of DAAs therapy in transplanted patients with HCV recurrence.