1. Background
Non-alcoholic fatty liver disease (NAFLD) refers to a group of liver abnormalities from steatosis to steatohepatitis, which can lead to cirrhosis and liver failure (1, 2). Moreover, NAFLD causes several pediatric serious comorbidities and behavioral problems (3, 4). Over the past three decades, with a significant increase in the prevalence of childhood obesity and overweight, the prevalence of pediatric NAFLD has increased (5), but because of the lack of reliable noninvasive screening tools, the real prevalence of NAFLD in children remains uncertain (6, 7). The global prevalence of NAFLD in children is about 7.6 and approximately 34% in obese children. Asia has the highest NAFLD prevalence, worldwide (8). Almost 20% of Asian obese children and adults have NAFLD, and it is more prevalent in the Middle East (9). Moreover, the NAFLD prevalence is over 30% in Iranian population (10). The prevalence of NAFLD in Iranian children has not recently been reported. Since Iran is one of the top ten countries in Asia with a high prevalence of obesity, the NAFLD prevalence in Iran is expected to increase in the future (9). In addition, given the industrial development and economic growth as well as lifestyle changes in developing countries like Iran, the rate of obesity, especially in children is rising (11). The prevalence of childhood obesity/overweight in the northwest of Iran is higher than that of its southern and central regions (12). Therefore, NAFLD is the most common liver abnormality in the first and second decades of life and its prevalence is increasing rapidly (13, 14).
2. Objectives
Accordingly, we conducted a large-scale study on pediatric NAFLD to evaluate its prevalence and predisposing factors in overweight and obese children aged 2 to 19 years in Urmia.
3. Methods
3.1. Study Population
In this cross-sectional study, we recruited 10800 children aged 2 - 19 years who were referred to the Digestive Disease Clinic of Shahid Motahari Hospital of Uremia University of Medical Science, between March 2016 and December 2017. Of the 10800 children, 843 samples (425 males) were eligible and agreed to participate in the study. A questionnaire was used for collecting the participants’ demographic information, medical history and alcohol consumption. Anthropometric and laboratory measurements and abdominal ultrasound for liver echogenicity and size were performed for the children. Inclusion criteria were children aged 2 - 19 years, the body mass index (BMI) of higher than 85th percentile, the absence of markers of hepatitis B virus (HBV) and C virus (HCV) in serum, and Wilson’s disease. The Ethics Committee of Urmia University of Medical Sciences approved our study protocol. Parents were informed about the study objectives and completed the written consent.
Transabdominal ultrasonography was performed by two trained and experienced radiologists using a Samsung ultrasound machine (Samsung Medison, Seoul, Korea) with a 5 to 7 MHz transducer probe for diagnosis of NAFLD. The ultrasound criteria for the diagnosis of NAFLD consist of Grade 1: a mild increase in liver echogenicity, Grade 2: a moderate increase in liver echogenicity and the limits of the diaphragm and intra-hepatic arteries are slightly faded, and Grade 3: a severe increase in liver echogenicity and the limits of the diaphragm and intra-hepatic arteries are severely faded and the posterior part of the right lobe is hardly observed. None of these symptoms was described as the normal liver (15).
3.2. Anthropometric Measurements
BMI was calculated as weight (kg)/height (m2). Z-scores of weight (Z-weight), height (Z- height) and BMI (Z-BMI) were calculated based on the World Health Organization (WHO) reference data for gender and age.
3.3. Biochemical Analysis
To measure blood glucose levels and serum lipids, venous blood samples were collected after 8 - 10 hours of overnight fasting. The samples were subsequently analyzed at a certified laboratory. Levels of serum Alanineaminotransferase (ALT), Aspartateaminotransferase (AST), and alkaline phosphate (ALK) were measured using commercial kits (Pars Azmoon, Tehran, Iran). Triglycerides (TG), cholesterol (Chol), low density lipoprotein (LDL) -cholesterol and high density lipoprotein (HDL)-cholesterol were also measured by enzymatic kits (Pars Azmoon, Tehran, Iran). Thyroid stimulating hormone (TSH) was measured by IRMA (Immunotech, Czech Republic). HBV surface antigens and HCV antibodies were measured by the enzyme-linked immunosorbent assay (ELISA) (Acon kit). HBsAg was detected using Biorad kits and anti-HCV was detected by Biomerieux kits. Fasting blood sugar (FBS) was measured enzymatically by auto-analyzer (Hitachi, Tokyo, Japan). Serum fasting insulin was measured using a chemiluminescent immunoassay method (DiaSorin, Liaison, Italy). The cutoff of ≥ 110 mg/dL was used for high FBS, ≥ 140 mg/dL for high TG, ≥ 190 for high total Chol and ≥ 115 mg/dL for high LDL levels. The cutoff of 440 U/L for elevated ALT levels and ≥ 20 mU/mL for high fasting insulin were used. According to the homeostatic model assessment of insulin resistance (HOMA-IR) method, Insulin resistance was calculated through the following formula:
Fasting glucose (mg/dL) × fasting insulin (mU/L) / 450.
3.4. Statistical Analysis
The quantitative variables were described as mean and 95% confidence intervals and qualitative variables were described using frequency (%). The normal distribution was examined by the Kolmogorov-Smirnov test. Student’s t-test and one-way analysis of variance (ANOVA) were used to compare the means in NAFLD and normal liver groups and the chi-square test was applied for categorical variables. Unconditional logistic regression was used to estimate the odds ratios (ORs) [95% confidence interval (CI)] for predictive factors of NAFLD. For adjusting potential confounding variables, the stepwise backward multivariable logistic regression analysis was done. Significant predictors of NAFLD at univariable analysis, considering clinically oriented confounder strategy were evaluated in three distinct multivariable logistic regression models. Model 1 consisted of age, gender, HDL-cholesterol, Z-BMI, ALT, AST, triglycerides, glucose, insulin and ALK. Model 2 consisted of age, gender, HDL-cholesterol, Z-BMI, ALT, AST and ALK and model 3 included age, gender, Z-BMI, FBS, TG, TSH, ALT, AST, glucose, insulin and ALK. In addition to gender, all predictors were evaluated as continuous variables.
4. Results
4.1. Characteristics of the Study Population
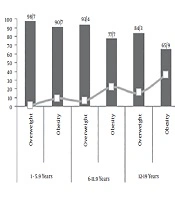
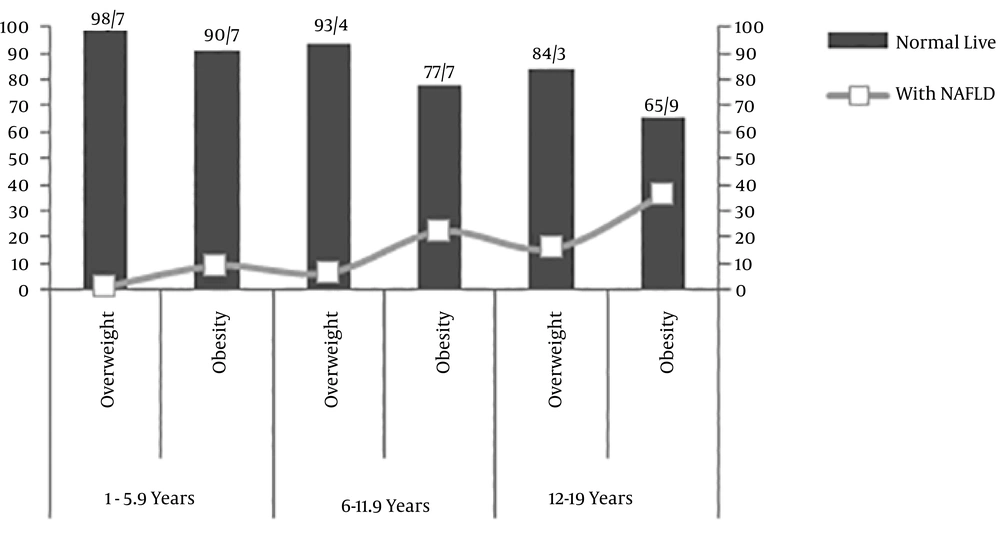
Of the 843 participants, 506 subjects (59.8%) were overweight and 337 subjects (40.2%) were obese. The percentage of subjects in the 2 - 5.9-year age group, 6 - 11.9-year age group and 12 - 19-year age group was 39.74 %, 48.87% and 11.39%, respectively. Eleven percent of the 843 children (n = 93) had NAFLD. The prevalence of NAFLD in overweight and obese children was 9.5 and 21.4 %, respectively. The prevalence of NAFLD in obese children was 9.3% for the age group of 2 - 5.9 years, 22.3% for the age group of 6 - 11.9 years and 35.6% for the age group 12 - 19 years (Figure 1). Among children with NAFLD, 65 cases (69%) showed grade one and 28 cases (31%) showed grade two. Table 1 represents the measurements of the children with and without NAFLD.
| Variables | Normal Liver (N = 750) | NAFLD (N = 93) | P Value |
|---|---|---|---|
| Gender (male/female, n) | 375/375 | 50/43 | 0.49 |
| Mean age | 7.3 [7.05 - 7.54] | 7.6 [7.36 - 7.83] | 0.766 |
| Z-weight (SDS) | 1.39 [1.33 - 1.46] | 1.54 [1.35 - 1.73] | 0.15 |
| Z-BMI (SDS) | 1.59 [1.54 - 1.64] | 1.66 [1.53 - 1.79] | 0.33 |
| FBS (mg/dL) | 83.43 [82.81 - 84.04] | 87.46 [85.84 - 89.07] | < 0.0001 |
| TG (mg/dL) | 103.3 [100.97 - 105.62] | 131.26 [117.62 - 144.89] | < 0.0001 |
| Chol (mg/dL) | 128.32 [125.91 - 130.73] | 159.6 [151.55 - 167.66] | < 0.0001 |
| LDL (mg/dL) | 107.59 [107.06 - 108.01] | 107.09 [103.15 - 111.02] | 0.63 |
| HDL (mg/dL) | 36.44 [36.07 - 36.81] | 39.13 [37.6 - 40.66] | < 0.0001 |
| TSH (µIu/mL) | 2.24 [2.14 - 2.34] | 2.73 [2.35 - 3.11] | 0.003 |
| Insulin (µIu/mL) | 12.73 [12.38 - 13.08] | 13.39 [12.33 - 14.46] | 0.223 |
| Cortisol (mcg/dL) | 16.22 [15.85 - 16.6] | 14.51 [13.18 - 15.83] | 0.013 |
| ALT (U/L) | 18.64 [18.11 - 19.17] | 35.54 [29.74 - 41.35] | < 0.0001 |
| AST (U/L) | 19.82 [19.41 - 20.23] | 29.85 [26.2 - 33.49] | < 0.0001 |
| ALK (U/L) | 295.33 [283 - 307] | 461.86 [398 - 524] | < 0.0001 |
| HOMA-IR | 2.6 [2.51 - 2.61] | 3.01 [1.09 - 8.7] | 0.022 |
| Age groups | < 0.0001 | ||
| 2 - 5.9 | 322 (96.12) | 13 (3.88) | |
| 6 - 11.9 | 356 (86.41) | 56 (13.59) | |
| 12 - 19 | 72 (75.0) | 24 (25) |
aValues are expressed as mean [95% confidence intervals] or No. (%).
4.2. Comparison of Children with and Without NAFLD
There was no statistically significant difference in the age of children with and without NAFLD (P = 0.766). There was no significant difference between females and males (P = 0.491). The levels of ALT (P < 0.0001), ALK (P < 0.0001), AST (P < 0.0001), triglycerides (P < 0.0001), cholesterol (P < 0.0001), TSH (P = 0.003), FBS (P = 0.0092) and HOMA-IR (P = 0.022) were higher in NAFLD children. No statistically significant difference was observed between two groups in terms of gender (P = 0.49), LDL-cholesterol (P = 0.63), insulin (P = 0.223), Z-weight (P = 0.15) and Z-BMI (P = 0.33).
Serum ALT was found higher than 30 U/L in 68 subjects (8%) and higher than 40 U/L in 35 subjects (25%), of whom 40 children in the former and 22 in the latter group had NAFLD (P = 0.025).
4.3. The Relation Between Biochemical Variables in Children with and Without NAFLD
In the adjusted logistic regression analysis, compared to the age group of 2 - 5.9 years, the odds of NAFLD was increased by 4 and 8 times in the age group of 6 - 11.9 and 12 - 19 years, respectively. An increase of 10 U/L in ALT, AST and ALK increased the odds of NAFLD 101%, 127% and 2%, respectively. An increase of 10 mg/dL in TG was associated with an 8% increase and also 10 µIu/mL of TSH with a 55% increase in the odds of NAFLD. An increase of 10 mg/dL in glucose was associated with a 34% increase in the odds of NAFLD. An increase of one unit in HOMA-IR was associated with a 19% increase in the odds of NAFLD (Table 2).
| Variables | Crude OR (95% CI) | Adjusted ORa (95% CI) |
|---|---|---|
| Model 1 | ||
| Age, y | ||
| 2 - 5.9 | 1 | 1 |
| 6 - 11.9 | 3.90 (2.09 - 7.26) | 4.89 (1.75 - 13.66) |
| 12 - 19 | 8.26 (4.01 - 16.99) | 9.56 (3.12 - 30.32) |
| Gender | ||
| Male | 1 | 1 |
| female | 0.86 (0.56 - 1.33) | 1.11 (0.55 - 2.22) |
| Model 2 | ||
| Insulin (µIu/mL) | 1.3 (0.85 - 1.97) | 0.98 (0.91 - 1.04) |
| HOMA-IR | 1.11 (1.02 - 1.25) | 1.19 (1.03 - 1.38) |
| Glucose (mg/dL) | 1.55 (1.24 - 1.92) | 1.34 (1.02 - 1.75) |
| Z-BMI (SDS) | 1.18 (0.85 - 1.65) | 2.7 (1.84 - 3.96) |
| Model 3 | ||
| TG (mg/dL) | 1.12 (1.08 - 1.19) | 1.08 (1.03 - 1.13) |
| Chol (mg/dL) | 1.22 (1.16 - 1.3) | 1.16 (1.09 - 1.23) |
| LDL (mg/dL) | 0.95 (0.76 - 1.18) | 0.92 (0.76 - 1.01) |
| Low HDL cholesterol (mg/dL) | 1.88 (1.39 - 2.46) | 1.58 (1.11 - 2.22) |
| TSH (µIu/mL) | 1.16 (1.66 - 14.3) | 1.55 (0.98 - 2.43) |
| ALT (U/L) | 2.22 (1.84 - 2.75) | 2.01 (1.64 - 2.46) |
| AST (U/L) | 2.34 (1.89 - 2.89) | 2.27 (1.81 - 2.86) |
| ALK (U/L) | 1.04 (1.03 - 1.06) | 1.02 (1.01 - 1.03) |
aModel 1 adjusted for age, gender, HDL-Cholesterol, Z-BMI, ALT, AST, triglycerides, glucose, insulin, and ALK; Model 2 adjusted for age, gender, HDL-Cholesterol, Z-BMI, ALT, AST and ALK; Model 3 adjusted for age, gender, Z-BMI, TSH, ALT, AST, glucose, insulin, and ALK.
5. Discussion
This study aimed to determine the prevalence of NAFLD in obese and overweight children and its association with biochemical parameters. Our results indicated that the prevalence of NAFLD in overweight and obese children was 9.5% and 21.4 %, respectively. Alavian et al. (16) reported the NAFLD prevalence in Iranian obese and overweight children aged 7 - 18 years 31.3% and 11.1%, respectively. This percentage is associated with the population’s characteristics as well as diagnostic techniques (17). In the present study and also Alavian study, ultrasonography was used as the diagnostic tool, however the rate of the age group of 12 - 19 years was less than that of younger children in our study. Forty percent of the children were younger than 6 years with a 3.8% prevalence of NAFLD, whereas 11.39% of the subjects were in the age group 12 - 19 years with an age-specific prevalence of 25%. More interestingly, the age-specific prevalence in overweight and obese children aged 12 - 19 years was 15.69 and 35.56, respectively. It should be noted that adolescent changes, such as hormonal changes in puberty, fat accumulation in the liver and more tendency to eat harmful foods may increase the prevalence of both obesity and NAFLD (18, 19). Various results have been reported in other studies, for example, in Germany, the NAFLD prevalence was 28% in obese children aged 8 - 19 years (20), in Brazil, it was 20.5% in obese children (21), in Turkey, a rate of 60.8% has reported in obese children aged 4 - 17 years (22) and in Mumbai, India its incidence has announced 12.9% in obese children aged 11 - 15 years (23).
In addition, in our study the prevalence of Grade 1, 2, and 3 fatty liver was 69, 31, and 0%, respectively. A similar research has been conducted in Iran reporting the 84.1% of mild, 14.3% of moderate and 1.6% of severe (16) grades of fatty liver. It should be noted that liver biopsy is the gold standard for assessing the severity of NAFLD (24). Ultrasonography may only predict mild grades. Recent studies have shown that children with slightly elevated ALT may have significant histological disturbances (25).
Our study indicated that the mean ALT, ALK and AST of children with NAFLD were significantly higher than the children without NAFLD. The increased levels of ALT and AST seem to be associated with NAFLD, clinically and histologically (26). These enzymes are commonly found in the liver cells and can enter the bloodstream due to liver damage, so the elevated levels of enzymes in the blood may be a marker for liver degeneration (27). Other studies verified the strong association between the elevated levels of liver enzymes and NAFLD (28, 29). Other studies also showed that ALT is associated with markers of oxidative stress and inflammation, which can lead to liver degeneration (30).
Interestingly, our study indicated that the NAFLD group had higher mean FBS and also they had high-insulin resistance, as estimated with an index of HOMA-IR. This is in agreement with the results of other studies (31, 32). Insulin prevents free fatty acid oxidation, and therefore, hyperinsulinemia may increase hepatotoxicity and steatosis by increasing free fatty acid in hepatocytes as well as generating free radical formation (33, 34).
Moreover, our results showed that TG might predict NAFLD in obese children. The obesity and accumulation and circulation of saturated fatty acids in the liver can result in liver degeneration by activating the apoptotic process (35). Free fatty acids are exposed to oxidative stress and by increasing β-oxidation can result in mitochondria degradation and the elevated levels of active oxygen is possibly a major cause for development of NAFLD (36). The pathogenesis of NAFLD can be introduced by a model, which is a two-hit theory, in which it is stated that the disease is caused by the first-hit due to insulin resistance, obesity and dyslipidemia, and due to the second-hits, such as oxidative stress, pro-inflammatory cytokines and intestinal bacterial toxins, ultimately inflammation and fibrosis of liver cells are occurred (37). On the other hand, liver enzymes, like ALT, as an indicator of oxidative stress and inflammation are factors resulting in insulin resistance (38).
Our findings revealed that TSH might predict NAFLD in obese children. The level of TSH has a remarkable effect on metabolism. Patients with overt hypothyroidism have the elevated total cholesterol and LDL-cholesterol levels (39, 40). In addition, NAFLD and hypothyroidism exert similar significant effects on metabolism, such as decreasing the fatty acid beta-oxidation and increasing lipid peroxidation. These changes are the leading source of oxidative stress and cell injury in the liver tissue (41). Regarding thyroid function, various studies have confirmed that the level of TSH in children with NAFLD is significantly higher than that of the control group and it is associated with an increase in the grade of NAFLD (39, 42). Our study results indicated that LDL-cholesterol can not predict NAFLD. Furthermore, we found that gender is not correlated with the prevalence of NAFLD.
To our knowledge, this is the first large-scale study on the prevalence of NAFLD and its predisposing factors in overweight and obese children of Urmia, Northwest of Iran. Despite our large sample size, we faced some limitations. First, its cross-sectional design cannot prove a causal relationship due to the absence of a normal-weight children group as well as the loss of patients for follow-up. Secondly, the gold standard for diagnosis is liver biopsy. The ultrasound cannot detect severe inflammation and fibrosis. However, we used ultrasound as a non-invasive, accessible and appropriate method for epidemiological studies with large sample size.
5.1. Conclusions
In conclusion, 21.4% of the obese children had NAFLD. Based on our findings, ALT, AST and HOMA-IR were associated with NAFLD and can predict the progression of the disease. Moreover, our findings indicated the importance of prevention of obesity and early intervention to prevent abnormalities to decrease morbidity among obese children. Further studies, perfectly executed with reference methods are needed for better determination of the status of obese children with NAFLD.