1. Background
Hepatitis B virus (HBV) infection remains a global public health problem, even following the development of an effective vaccine. Brazil is classified as a region with low to intermediate prevalence of HBV surface antigen (HBsAg) carriers (< 2%), including areas of high HBV endemicity (> 8%) (1). Occult HBV infection (OBI) is the most challenging issue in the field of viral hepatitis, with its virological and clinical relevance in patients undergoing treatment for hepatitis C being a hot topic of debate at present. Occult HBV infection is determined by the absence of HBsAg and presence of HBV DNA in liver or serum of infected patients (2, 3) and classified as: (1) seropositive OBI (positive for antibody to hepatitis B core antigen [anti-HBc] and/or antibody to hepatitis B surface antigen [anti-HBs]), (2) seronegative OBI (negative for anti-HBc and anti-HBs), and (3) “false” OBI due to the presence of HBV variants with mutations in the S gene (escape mutants) producing modified HBsAg that are not recognized by some or all commercially available detection assays (2, 3). The frequency of OBI in Brazil ranges from 0% to 24% (4-9) and has significant relevance in a clinical context, since it can lead to the development of severe hepatic diseases, such as cirrhosis and hepatocellular carcinoma, and eventually, death. Viral factors of OBI induction may be associated with mutations, especially in the S protein, and co-infection with other viruses such as hepatitis C virus (HCV) (10). Reactivation of HBV has been reported in patients with chronic HCV or resolved HBV infection under treatment with newer direct-acting antivirals (DAA) (11), resulting in fulminant hepatitis, liver failure and in some cases, death. According to the Brazilian Clinical Protocol and Therapeutic Guidelines for Hepatitis C and Coinfections, individuals detected with HBsAg prior to initiation of DAA are recommended HBV therapy to prevent reactivation owing to hepatitis C treatment. However, in cases where HBsAg is not detected, treatment with DAAs is released without examining for the presence of HBV-DNA (12). Although hepatitis B reactivation in patients is known to occur during hepatitis C treatment with direct-acting antivirals, only a few cases have been described to date. Moreover, no data are available on OBI/HCV coinfection in patients scheduled for DAA treatment in Brazil.
2. Objectives
In this study, we evaluated the prevalence of anti-HBc and examined for OBI via real-time and nested PCR analyses in patients with chronic hepatitis C before commencement of DAA therapy. We additionally investigated the presence of mutations in the S gene associated with cases of false OBI.
3. Methods
Our retrospective cross-sectional study was conducted in patients prior to commencement of DAA treatment who had attended the Outpatient Clinic of Liver Disease at the Gaffrée and Guinle University Hospital (Rio de Janeiro, Brazil) from January to December 2018. Clinical, demographic as well as HBV and HCV treatment data were acquired from medical records. Serum samples were collected from a cohort of 114 consecutive HCV patients. The patients included for study had failed pegylated interferon and ribavirin (PEG-INF and RBV) therapy. All serum samples were HCV RNA-positive and examined for total HBc and HBsAg via immunoenzymatic assays (EIA). Samples positive for anti-HBc with no HBsAg were further examined using real-time PCR (qPCR) and nested PCR (13). The HBV DNA was additionally sequenced for genotyping and detection of S gene mutations. Notably, the presence of OBI was correlated with aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels.
4. Results and Discussion
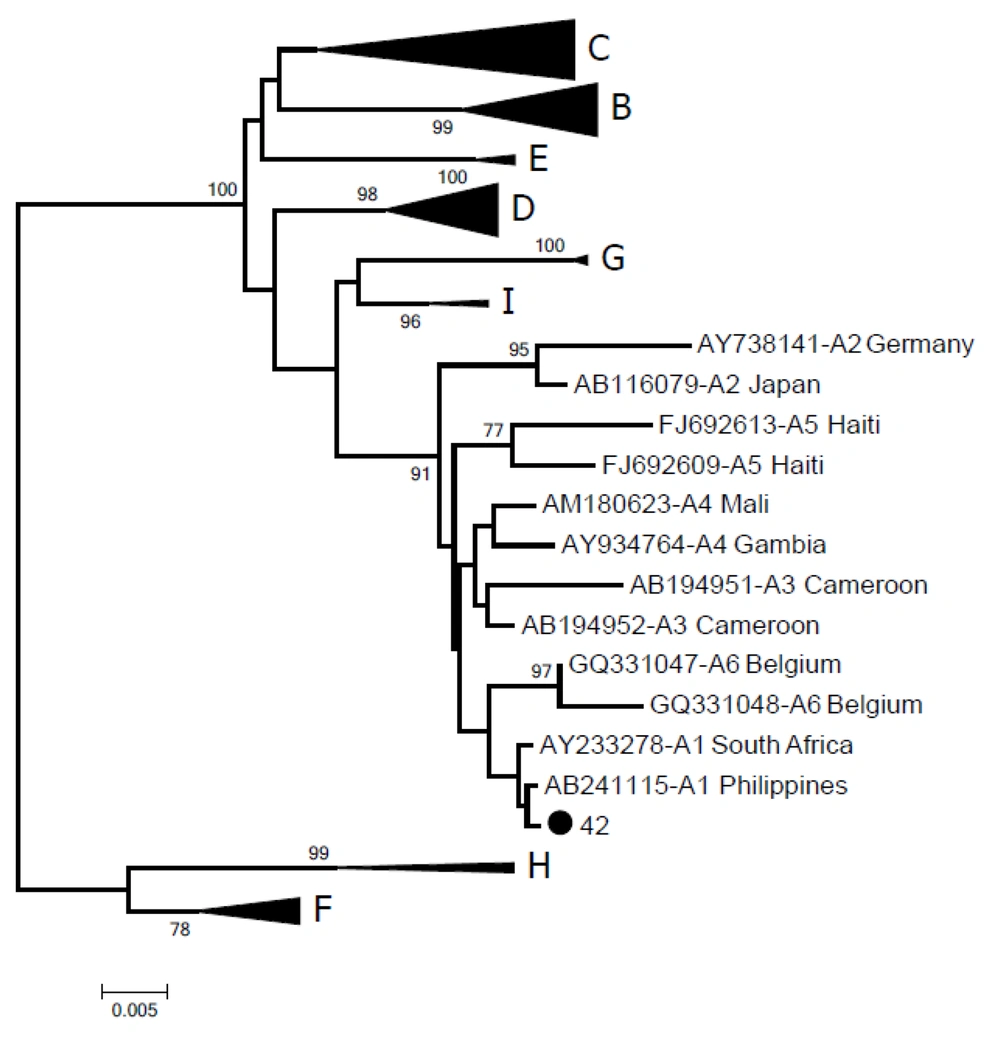
In a serological test, 37.7% (43/114) patients were identified as positive for anti-HBc, consistent with a previous report of antibody prevalence of up to 35% in HCV-infected populations (11). Occult HBV infection was detected in three (2.6%) male patients with a mean age of 69.3 years classified as HCV genotypes 1 and 3. The patients had received treatment with pegylated interferon (80 mcg) and ribavirin (1.0 g per day) for 24 weeks without HCV clearance. No patients developed ALT and AST flare-ups and all were anti-HB-positive, with viral loads ranging from 207.14 to 1547 IU/mL (1.16 × 103 to 8.51 × 104 copies/mL) (Table 1). Patients with the highest viral loads were positive for S-region amplification (GenBank accession number MK360178) via nested-polymerase chain reaction (nested-PCR) and HBV in these cases was classified as genotype A1 (Figure 1), the most prevalent in Brazil (14). Deletion or insertion of nucleotides in the S region was not associated with non-detection of HBsAg with commercial kits or non-expression of HBV surface antigen. The patient had chronic active hepatitis C, grade 4, with cirrhosis (F4, 38.5 kPa; fibroscan). All patients were re-treated with sofosbuvir (400 mg) + daclatasvir (60 mg) + ribavirin (1.0 gram), following which HCV remained undetectable. The presence of HBV DNA has been reported among patients with hepatitis C treated with DAAs. However, in patients with resolved (anti-HBs and anti-HBc) HBV infections, no HBV reactivation-related hepatitis is documented (3). In the current study, all three patients had HBV DNA viral loads < 2000 IU/mL before DAA therapy. A systematic review and meta-analysis published in 2018 provided evidence of significantly lower relative risk of HBV reactivation-related hepatitis in patients with HBV DNA below the lower limit of quantification at baseline than those with quantifiable HBV DNA (11).
| ID | Copies/mL | IU/mL | ALT | AST | HCV Genotype | HBV Genotype | Fibrosis Stage |
|---|---|---|---|---|---|---|---|
| 001 | 1.16 × 103 | 207.14 | 59 | 102 | 1a | ND | F4 |
| 042 | 8.5 × 104 | 1517.8 | 87 | 137 | 3a | A1 | F4 |
| 268 | 1.29 × 104 | 230.357 | 74 | 100 | 1b | ND | F4 |
Viral Load of HBV, Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT) Levels in Patients Infected with HCV and Occult Hepatitis B
Phylogenetic tree of the HBV S-gene inferred using the neighbor-joining method. Values in the branches indicate the percentage of 1000 bootstrap replicates supporting the group. The sequence generated in this study is represented by a black dot (GenBank accession number MK360178). Reference sequences are indicated by their accession number, followed by subgenotype and geographical origin.
Since the patient populations at risk for HBV reactivation and management options are currently unclear, these cases clearly highlight the importance of HBV DNA testing prior to initiation of DAA therapy. Several studies assessing the prevalence of OBI in patients with chronic HCV infection have reported dissimilar rates of HBV DNA positivity, which may be explained by differences in the prevalence of HBV infection in different geographic regions, detection limits of HBV DNA assays and/or the biological material analyzed (liver tissue or serum) (9). Low levels of anti-HBs and decrease to < 12 mIU mL after treatment are significant risk factors for HBV reactivation or reappearance (15). We observed no differences in anti-HBs levels before and after treatment with DAAs and the small elevation of ALT/AST observed in patients with OBI HBV reactivation could be prevented with pretreatment screening and prophylactic treatment where necessary (16). It can occur spontaneously but is triggered by various factors and often transient without clinical symptoms in most cases other than causing a hepatitis flare. Moreover, HBV reactivation may occur regardless of HCV genotype and type of DAA regimen (16).
While OBI and risk of HBV reactivation are relatively rare, it is important to account for the possibility that in some instances, subjects undergoing new treatments are infected with HBV viral DNA, since HCV and previous treatments can inhibit HBV replication and influence evolution of the disease. Based on findings from the current study, we recommend that clinicians should be aware of possible anti-HBc positivity in patients and examine for hepatitis B infection along with the issues surrounding OBI screening before initiating treatment with novel DAAs. To our knowledge, this is the first study in Brazil to focus on OBI in individuals with chronic hepatitis C before DAA treatment. Our collective results should contribute to formulating effective therapeutic guidelines for treatment of hepatitis C and concomitant infections and support routine evaluation of OBI/HCV coinfections before and during treatment with DAAs.