1. Background
Liver cirrhosis is a histopathologic entity, which is defined as the damaged hepatocytes and their replacement with fibrotic tissue. Several factors are involved in cellular damage and liver cirrhosis, including chronic viral hepatitis, autoimmune hepatitis, nonalcoholic steatohepatitis (NASH), metabolic disorders etc. Liver cirrhosis is considered as cryptogenic cirrhosis in cases, where the reason for liver damage cannot be defined or determined (1, 2).
Nonalcoholic fatty liver (NAFLD) is caused by a build-up of fat in the liver in the absence of significant alcohol use, which nowadays, is one of the most common liver disorders. It is commonly associated with one of the components of metabolic syndrome, including glucose intolerance, central obesity, hypertension, hypertriglyceridemia and low levels of HDL. One end of a spectrum of this disorder is simple accumulation of fat in the liver and steatosis, whereas the other end can result in liver cirrhosis that is categorized as cryptogenic cirrhosis (3-8). In this condition, fat accumulation activates oxidative stress and lipid peroxidation, which in turn trigger inflammatory process and cytokines induction leading to liver fibrogenesis (8, 9).
Statins are HMG-CoA reductase inhibitors that are widely used in the management of lipid disorders. Different studies have shown their positive effects in management of dyslipidemia and also their anti-inflammatory effect, which can decrease in vitro production of inflammatory cytokines. Based on these findings, several studies have evaluated the efficacy of atorvastatin in regression of liver fibrosis and prevention of liver cirrhosis with some promising results (8-10). Aspirin (ASA) is one of the derivatives of salicylic acid with anti-inflammatory and anti-platelet properties that has evaluated for reduction of liver inflammation and fibrosis based of the potential role of platelets in the inflammation process and fibrosis induction (11-13).
2. Objectives
According to the findings of previous studies (9, 11, 14) and also increasing prevalence of obesity and NAFLD as progenitors of cryptogenic cirrhosis, the current clinical trial was designed to evaluate the efficacy of a combination of ASA and Atorvastatin in improvement of liver fibrosis and function among patients with cryptogenic cirrhosis.
3. Methods
In this randomized double-blind clinical trial, 40 patients with cryptogenic cirrhosis who were attended the Outpatient Liver Clinic of Qom University Hepatology Research Center divided into two groups. The Inclusion criteria included the age range of 18 - 75 years, no history of treatment with statins, serum LDL level of more than 70 mg/dL and platelet count of more than 50,000. The participants were excluded in case of more than 3 times elevation of liver transaminases 3 months after start of trial, prove of noncompliance during follow up, statin induced myopathy, current consumption of any tablet from fibrate group, erythromycin or any other inhibitor of P450 enzyme metabolism, history of GI bleeding or esophageal varices, liver stiffness ≥ F3 in Fibroscan® or stigmata of recent gastroesophageal variceal bleeding. All patients were on low salt (less than 2 g per day) diet.
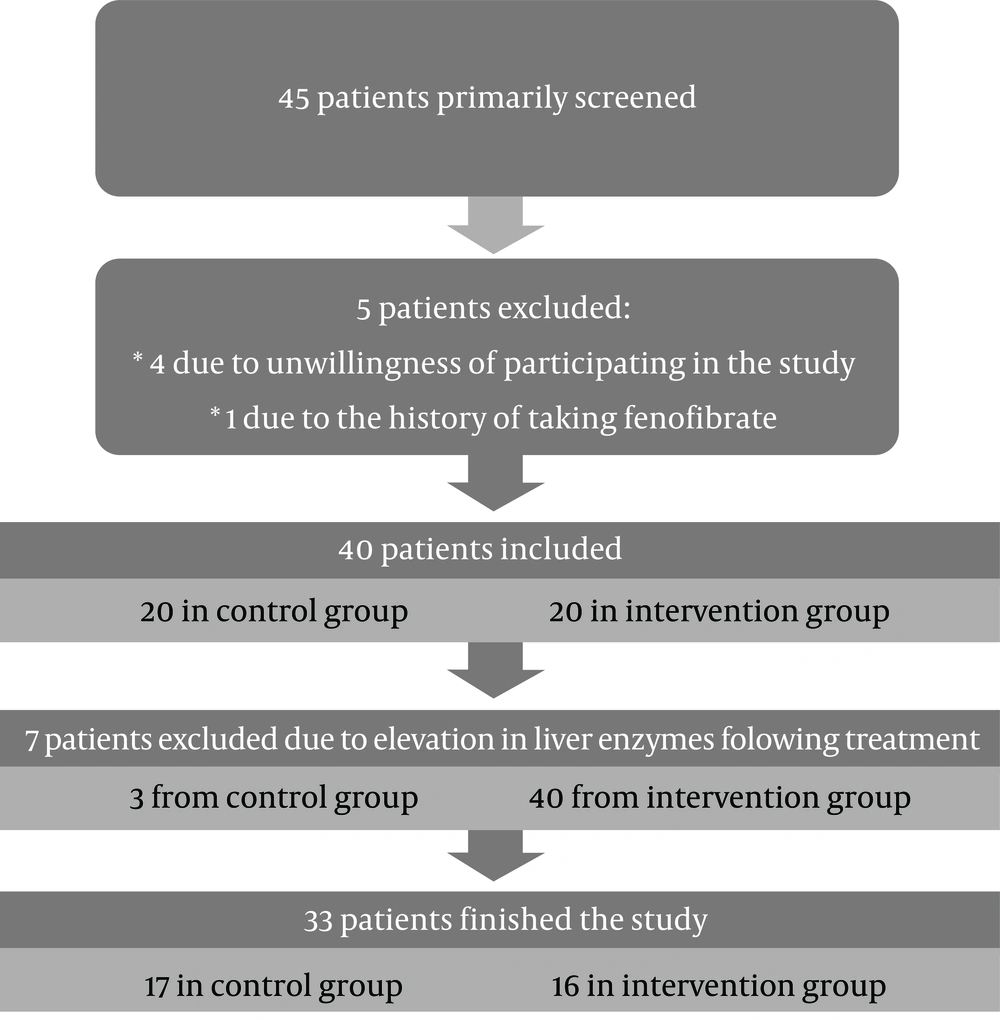
At the beginning, 45 patients screened from which 4 patients excluded due to unwillingness of participating in the study and one excluded due to the history of using Fenofibrate. Figure 1 shows study flowchart.
The participants were informed about the research objectives and the signed informed consent was obtained. The demographic characteristics and laboratory findings of the patients, including degree of liver stiffness were recorded as the baseline data. The patients then were randomly allocated to the intervention or control group by block randomization. The size of each block was 4. Patients allocated to the intervention group (group A) treated with 20 mg atorvastatin plus ASA 80 mg daily and patients allocated to the control group (group B) received 20 mg atorvastatin plus placebo daily. Following a six-month intervention, the liver biochemical profiles as well as the degree of liver stiffness were re-measured and recorded. The levels of liver stiffness were measured by Fibroscan® (FibroScan 402, Echosense, France). To maintain blinding of the investigators, a code number was assigned to all medications and were delivered by a physician blinded to the trial. This study was approved by the Qom University Ethics Committee (approval code: IR.MUQ.REC.1395.134) and registered at the Iran Portal of Clinical Trials (Research ID: IRCT2016120531252N1).
The recorded results were analyzed by SPSS version 24 using chi-square test and paired t-test. P values of less than 0.05 were determined as significant.
4. Results
Overall, 3 patients in the control group and 4 patients in the intervention group were excluded due to liver transaminases elevation above 3 × ULN or noncompliance and finally 16 participants in group A and 17 in group B evaluated for final analysis. The mean age of patients in groups A and B was 50.3 ± 11.2 and 47.9 ± 10.6 years, respectively. The average body mass index (BMI) of the participants in two groups was 30.7 ± 4.2 and 30.8 ± 3.1, respectively. Twelve patients in group A (75%) and 14 cases in group B (82%) were male. No significant difference was found in both groups in terms of demographics including age (P = 0.527), BMI (P = 0.983) and sex (P = 0.688).
Following a six-month intervention, the average alanine aminotransferase (ALT) level significantly decreased in group B (atorvastatin + placebo) (P = 0.009), whereas it was not significant in group A (atorvastatin + ASA). The average Child score of participants in both groups was improved significantly (P = 0.0001 and 0.002, for A and B respectively) and also the liver stiffness measurement by Fibroscan proved a significant decrease in liver fibrosis in groups A and B (P < 0.001 and P = 0.007, respectively) (Table 1). Despite a significant decrease in degree of liver stiffness and child score, there was not any significant difference between 2 groups (P = 0.982 and 0.611, respectively). Comparing the mean level of liver transaminases in both groups after the treatment demonstrates no significant difference in the mean level of SGOT between the groups (P = 0.506) but the level of SGPT was significantly lower in group A (atorvastatin + ASA) (P = 0.007).
| Group 1 (Atorvastatin + ASA) | P Valuea | Group 2 (Atorvastatin + Placebo) | P Valuea | |
|---|---|---|---|---|
| SGOTb | 0.65 | 0.2 | ||
| Before | 44.87 ± 19.54 | 39.05 ± 15.68 | ||
| After | 43.75 ± 16.59 | 40.24 ± 13.33 | ||
| SGPTc | 0.74 | 0.009 | ||
| Before | 44.12 ± 23.46 | 41.76 ± 16.65 | ||
| After | 44.94 ±20.99 | 47.35 ± 14.21 | ||
| Child score | < 0.001 | 0.002 | ||
| Before | 7.50 ± 1.41 | 6.59 ± 1.22 | ||
| After | 5.87 ± 0.88 | 5.88 ± 0.99 | ||
| Fibroscan, Kpa | < 0.001 | 0.007 | ||
| Before | 36.06 ± 20.72 | 27.92 ± 18.30 | ||
| After | 28.79 ± 19.74 | 25.35 ± 18.71 |
aBased on the paired t-test.
bSerum glutamic-pyruvic transaminase.
cSerum glutamic oxaloacetic transaminase.
5. Discussion
Based on the results, the average levels of serum ALT, Child score and liver stiffness were similar between 2 groups, whereas after the intervention period, there was a significant decline in serum ALT in group B (atorvastatin + placebo). The level of ALT in group A and level of serum AST in both groups did not show any significant decrease (P > 0.05). The findings of Gomez et al. study indicated that management of patients with NAFLD using Atorvastatin could result in improvement and normalization of liver ALT (15), however this effect in the current study was only observed for ALT and after treatment with Atorvastatin and ASA no significant effect was seen. This difference between two studies cab be explained by different study samples, as in our trial, only cirrhotic patients were included.
The average child score of participants in both groups showed a decrease after intervention, however it was not significant and it can be concluded that Atorvastatin has a positive effect on liver function among patients with cryptogenic cirrhosis, whereas adding ASA to this therapeutic regimen had not a positive synergistic effect.
The results of Fibroscan indicated a significant decrease after the intervention in both groups, whereas there was no significant difference between groups A and B. This finding shows the positive effect of management with Atorvastatin and non-effectiveness of adding ASA. A systematic review by Kamal et al. in 2017 (14) showed the positive effect of Atorvastatin in liver function and fibrosis, which is consistent with our results. Jiang et al. (11) in 2016 reported that prescription of ASA for patients with chronic liver disease could result in improvement of liver fibrosis and also Li et al. (16) revealed this efficacy on liver fibrosis in animal models, which are not consistent with the present study. This difference can be due to the inclusion criteria of these studies, in which all degrees of liver fibrosis were studied and also the samples with different etiologies, including chronic viral hepatitis, alcoholic liver disease (ALD) and or non-alcoholic steatohepatitis (NASH) were included. Accordingly, designing future studies with more specific inclusion criteria as well as evaluating subpopulation of samples with equal degrees of liver fibrosis and common etiology can more clarify this issue.
Simon et al. (17) in a prospective study on 151 aspirin user with biopsy-confirmed NAFLD concluded that daily routine use of aspirin, improves the indices of liver fibrosis including FIB-4 and transaminases to platelet ratio. These findings are not compatible to our study. However, researchers of the mentioned study stated that aspirin should be taken daily at least for four years to be effective on improvement of liver fibrosis. In our study, short term treatment with aspirin was not effective for reducing liver fibrosis.
Results from a meta-analysis which published in 2019 by Iqbal et al. (18), analyzed the findings of 4 clinical trials with summed population of 2310 patients to discover the effect of aspirin use on liver fibrosis. Although the results show that routine use of aspirin will decrease odds of liver fibrosis, researchers stated that further clinical trials are necessary, with respect to the limited number of studies and pooled population.
Singh et al. (19) in a cohort study on 592 patients with type 2 diabetes mellitus and NAFLD (that approved by liver biopsy) who were using routine aspirin, concluded that aspirin has not any significant effect on prevention or reducing liver fibrosis in patients with NAFLD, which was similar to our results.
Overall, based on the results of this study and compared to other evaluations, it can be concluded that Atorvastatin has noticeable effect on improvement of liver fibrosis and subsequently liver function among patients with cirrhosis and a combination of ASA and Atorvastatin has the same effect without any additive effect, at least in short-term treatment. It seems that ASA has no role in potentiation of Atorvastatin efficacy in improvement of liver fibrosis and function among cases with compensated cryptogenic liver cirrhosis. On the other hand, based on the absence of significant side effects with consumption of ASA and multiple advantages of this medication, including being cost-effective and also its availability and cardio-protective effects, ASA can be recommended especially in early stages of liver cirrhosis. It should be considered that several patients with NAFLD and cryptogenic cirrhosis, have also other criteria of metabolic syndrome with the increasing risk of cardiovascular disease (CVD) or cerebrovascular accident (CVA) and they potentially would benefit from ASA prescription. Besides, there are some evidences that shows long-term and routine use of aspirin has positive effect on prevention and reduction of liver fibrosis.
5.1. Conclusions
Based on the findings of current study, it can be concluded that although Atorvastatin is effective in improvement of liver fibrosis and function in patients with cryptogenic cirrhosis, adding ASA is unable to potentiate its positive effects in short-term treatment, however it is not still clear that combination of ASA and Atorvastatin in long-term follow up would increase the effects of Atorvastatin or not? Further clinical trials with longer duration of follow-up will answer this question.