1. Background
Ulcerative colitis (UC) is a chronic inflammatory bowel disease with unknown etiology. The clinical manifestations of the disease mainly include hematochezia and abdominal pain. A Mayo score (1) of ≥ 11 points classifies UC as acutely severe. Before the use of hormones, the mortality rate of ASUC patients ranged from 22% to 75% (2). Following hormone administration, 20% - 40% of patients still require immunosuppressant therapy, which not only leads to severe side effects, but also represents an expensive form of treatment. Furthermore, some patients still require surgery, which may represent a great psychological and physical burden. Therefore, it is particularly critical to identify other effective pathophysiological treatment approaches (3-5).
In recent years, a number of studies have revealed that the blood of UC patients is often in a hypercoagulantion state (6). Furthermore, the incidence of asymptomatic deep vein thrombosis (DVT) without symptoms is as high as 11% (7). Pathological mucosal biopsies obtained via colonoscopy have revealed that the positive rate of microthrombus was 20% - 30% in the lesion area of UC patients. Additionally, the positive rate of surgical resection specimens was reported to reach 66.7% (8, 9). This may occur due to intestinal mucosal necrosis prompting the body to release a large number of inflammatory factors, thereby activating the coagulation system and inhibiting the anticoagulant and fibrinolytic systems, resulting in blood hypercoagulation. This hypercoagulability also causes local intestinal mucosal capillary occlusion and microthrombus formation, leading to tissue ischemia, hypoxia, necrosis, inflammation, and ulcer formation. Inflammation interacts with hypercoagulability, leading to a vicious disease cycle, which blocks drug absorption and further aggravates hormone resistance and tolerance. Therefore, it is imperative to determine the effect of anticoagulation therapy on patients with ASUC.
According to consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease (IBD): Canadian Association of Gastroenterology (10) in 2014, inpatients with IBD but without active massive bleeding are recommended receiving anticoagulant therapy. Furthermore, low-molecular-weight heparin (LMWH) or low-dose ordinary heparin may be administered to prevent thrombosis and anticoagulant therapy is recommended for three months after remission. Although heparins can prevent thrombosis, the improvement of UC clinical prognosis is uncertain (11-13). Some studies have determined that oral LMWH enhanced the repair capacity of intestinal mucosa by increasing the local drug concentrations in the intestinal tract, resulting in a clinical remission rate of up to 70% (14-18). However, such drugs are still in the experimental stage. Therefore, a new oral anticoagulant is urgently required for clinical application. Rivaroxaban is a Non-vitamin K antagonist oral anticoagulant (NOAC) that selectively blocks the Xa factor of the active site; it also does not require cofactor activity, acts quickly, has a short half-life, interacts less, dose-effect of drug changes little and produces a relatively low rate of intracranial hemorrhage.
2. Objectives
In the present study, hemortheology tests were performed on ASUC patients to determine the presence of blood hypercoagulability and to explore the effect of rivaroxaban anticoagulation therapy.
3. Methods
3.1. Patients
From April 2016 to April 2018, the study enrolled 88 ASUC patients admitted to the Department of Gastroenterology, Affiliated Hospital of Qingdao University. We also collected 40 colonic polyps patients for comparison. The ASUC patients were randomly divided into control and treatment groups (n = 44 each). All the UC patients were confirmed via electronic colonoscopy and pathology to meet the diagnostic criteria for acute and severe disease. We excluded patients diagnosed with Crohn’s disease, infectious colitis, ischemic colitis or radiation colitis. We also excluded patients who had clotting disorders, severe liver disease, and kidney failure or those who were in pregnancy or breast-feeding. The administration of glucocorticoids or other immunosuppressive drugs were prohibited for the first four weeks of the current study. The patient was withdrawn from the study, if no clinical improvement was observed, disease progression occurred or the patient exhibited significant side effects, including elevated aspartate transaminase/alanine aminotransferase (AST/ALT) within four weeks of treatment. The baseline demographic and clinical characteristic of ASUC patients are presented in Table 1. The two groups were comparable in terms of sex, age, Montreal classification (19), Mayo score , UCEIS score (20) (Supplementary file appendix 1), Geboes index classification (21) (Supplementary file appendix 2) and other aspects (P > 0.05). All volunteers provided their written informed consent before enrollment. The current study was approved by the Research Ethics Committee of the Affiliated Hospital of Qingdao University.
| Control Group | Treatment Group | |
|---|---|---|
| Gender (n) | ||
| Male | 29 | 27 |
| Female | 15 | 17 |
| Age, y | ||
| Minimum age | 21 | 76 |
| Maximum age | 20 | 78 |
| The average age | 50.57±12.3 | 48.02±15.07 |
| Montreal classification (n) | ||
| E2 | 7 | 7 |
| E3 | 37 | 37 |
| Mayo score (n) | ||
| 11 score | 41 | 41 |
| 12 score | 3 | 3 |
| UCEIS score (n) | ||
| 6 score | 38 | 37 |
| 7 score | 6 | 7 |
| Geboes index (n) | ||
| Level 4 | 17 | 17 |
| Level 5 | 27 | 27 |
Baseline Demographic and Clinical Characteristic of ASUC Patients
3.2. Detection Methods
We tested 88 ASUC patients and 40 colonic polyps patients for hemorheology to assess the following indicators: Mean platelet volume (MVP; normal range, 7.4 - 11.0 fL), platelet count (normal range, 125 - 350 × 109 /L), Prothrombin Time (PT; normal range, 10.5 - 14.5 seconds), activated partial thromboplastin time (APTT; normal range, 28 - 43.5 seconds), Fibrinogen (FIB; normal range, 2 - 4 g/L), D-dimer (D-D; normal range, 0 - 500 ng/L), and Thromboelastograghy (TEG index: R, normal range, 5 - 10 minutes; K, normal range 1 - 3 minutes; α angle, normal range, 53 - 72 degrees; MA, normal range, 50 - 70 mm; CI, normal range, -3 - 3).The R value was measured as the time from the beginning of the test to the amplitude reaching 2 mm, which reflected coagulation factor activity. The K value referred to the time from the end of the R value to the amplitude reaching 20 mm, which indicated the time and rate of blood clot formation. The MA value indicated the maximum amplitude of the image, demonstrating the maximum strength of the clot, which is related to platelets and fibrinogen and greatly affected by platelets (accounting for 80%). The α angle (Angle) between the curve tangent and the horizontal line at the image opening was also determined, which indicated the function of fibrin. The CI value was calculated according to the R, K, Angle and MA values and was used to indicate the comprehensive coagulation index. Hypocoagulability is indicated, which when less than -3; hypercoagulability is indicated, which when more than 3. The higher the blood viscosity, the smaller the R and K values, the larger Angle, MA and CI values. The anticoagulant blood was sampled from patients after an overnight fast; 2 mL of blood was injected into a heparin anticoagulant tube, a further 2 mL was injected into a tube for routine blood tests, and 7.5 mL was injected into a tube for TEG test. The following detection devices were utilized: An automatic blood coagulator (Beckman Coulter, Inc.), blood routine instrument (Beckman Coulter, Inc.) and TEG 5000 Thromboelastograph hemostasis analyzer (Haemonetics Corporation, Skokie, Illinois, USA). The aforementioned indicators were reviewed in patients with ASUC 30 days later and compared with the data obtained prior to treatment.
Biopsies were taken at sites where inflammation was most prevalent. If no inflammation was observed, biopsies were performed at random sites. Biopsy specimens were fixed with formalin, embedded in paraffin and sectioned with hematoxylin and eosin staining. Biopsy specimens were then reviewed by a double-blind pathologist and graded using the Geboes index. The most severe inflammatory activity was merely recorded and biopsy specimens were reassessed to determine the consistency of pathological criteria. The Geboes score ranged from 0 to 5.4, with higher scores indicating more severe inflammation. The Geboes scores ≥ 3.1 were defined as histological inflammation indicative of active UC stages. Microthrombus was defined as a thrombus that occurred in the venules, arterioles and capillaries of the microcirculation. This could only be identified under a microscope and was composed of platelets and cellulose, without red blood cells. Immunohistochemical microthrombus staining for intestinal mucosa pathology was performed using mouse anti-human CD61 antibodies. To determine CD61 positive ratio in the tissue, optimal cutting temperature (OCT) sections were fixed, quenched, blocked, and then incubated with primary antibody CD61, secondary antibody (biotinylated goat anti-mouse IgG), and avidin-biotin-peroxidase complex. Following staining with 3,3′-diaminobenzidine solution, counterstaining was performed with hematoxylin. Finally, the sections were dehydrated and fixed. When the percentage of microthrombus was counted, two pathologists who were not aware of the experimental protocol confirmed the count, and 10 fields were randomly selected for each section. Microcirculation vessels with an outside diameter ranging from 50 to 100 μm were observed randomly and the percentage (%) of blocked vessels was calculated. The average value was subsequently obtained.
3.3. Treatment Strategy
In the control group, patients with ASUC were routinely treated with oral mesalazine ( Etiasa®, 500 mg, Ipsen Pharmaceutical Companies, France) 1 g administered four times per day and intravenous Methylprednisolone Sodium Succinate (Methylprednisolone®, 40 mg, Pfizer Manufacturing Belgium NV) 40 or 60 mg once per day. In the treatment group, in addition to the routine treatment regimen, patients with ASUC received oral rivaroxaban (Xarelto®, 10 mg, Bayer Pharma AG, Germany, and Janssen Pharmaceuticals, Inc., New Jersey) 10 mg once per day for 30 days. The treatment was terminated if patients exhibited bleeding tendency. After being discharged from the hospital in a stable condition, patients received oral methylprednisolone tablets (Medrol®, 4 mg, Pfizer Italia S.r.l.). No contraindication was identified in patients for rivaroxaban.
3.4. Efficacy Evaluation Criteria
The Mayo scoring system was used to evaluate the activity and treatment efficacy of the disease. Additionally, the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) was used to evaluate mucosal inflammation and the Geboes index was used to evaluate the mucosal histology. The effective definition is that the reduction of Mayo score relative to the baseline value is ≥ 30% and ≥ 3 points, and the sub score of stool blood is ≥ 1 point or the sub score is 0 or 1 point. 30 days later, the two groups were compared for the efficacy and side effects and the percentage of micro thrombus in pathological sections were also compared before and after treatment.
3.5. Statistical Analysis
Statistical analysis was performed using SPSS version 16.0 software. Data conforming to normal distributions were represented by
4. Results
4.1. The Indexes and Comparison of Hemorheology in All Patients
The results revealed that the indices of ASUC patients were significantly different from those of colonic polyps patients (P < 0.01). Specifically, MVP (9.119 ± 1.1633 vs. 10.112 ± 0.5788), R (3.919 ± 0.7415 vs. 5.142 ± 0.5737) and K (1.292 ± 0.3016 vs. 0.976 ± 0.8716) values were lower in the ASUC patient group than in the colonic polyp group. Furthermore, platelet count (437.98 ± 95.912 vs. 210.8 ± 39.236), APTT value (31.786 ± 5.0735 vs. 28.115 ± 3.1806), PT value (12.303 ± 1.5152 vs. 11.392 ± 0.7943), FIB value (4.0362 ± 1.5783 vs. 2.7445 ± 0.50162), D-dimer value (878.95 ± 265.323 vs. 285.5 ± 105.562), Angle value (77.192 ± 2.1154 vs. 70.908 ± 3.3227), MA value (68.811 ± 5.5366 vs. 60.97 ± 4.7622) and CI value (3.62 ± 0.8252 vs. 1.208 ± 0.9558) were higher in ASUC patients than the colonic polyp group.
4.2. The Indexes and Comparison of Hemorheology in ASUC Patients
The platelet count (253.27 ± 50.113 vs. 432.93 ± 80.090), FIB (3.0602 ± 0.65374 vs. 4.0261 ± 1.1061) and D-dimer (299.84 ± 125.23 vs. 878.52 ± 278.915) values were significantly reduced after treatment in the control group of ASUC patients (P < 0.01). However, no statistically significant different was observed in MVP (9.395 ± 0.9104 vs. 9.302 ± 1.1230), APTT (32.975 ± 4.9762 vs. 31.884 ± 4.9395) and PT (12.920 ± 1.2212 vs. 12.482 ± 1.7116) values before and after treatment in the control group of ASUC patients (P > 0.05). While the platelet count (248.8 ± 62.714 vs. 433.02 ± 110.207), FIB (3.1661 ± 0.72088 vs. 4.0464 ± 1.22003) and D-dimer (352.27 ± 169.375 vs. 879.39 ± 254.236) values significantly reduced (P < 0.01), and the values of APTT (34.977 ± 6.1549 vs. 31.689 ± 5.2593) and PT (13.459 ± 1.1809 vs. 12.125 ± 1.2846) increased (P < 0.05) after treatment in the treatment group of ASUC patients. However, the final values of APTT and PT were both within the normal range, and no differences were observed in MVP (9.177 ± 0.9425 vs. 8.936 ± 1.1868) value (P > 0.05) in the treatment group of ASUC patients. Furthermore, differences between MVP, APTT, PT, platelet count, FIB an D-dimer values were not statistically significant before and after treatment in both groups (P > 0.05).
The R (3.702 ± 0.7763 vs. 4.439 ± 0.8139; 4.118 ± 0.6650 vs. 5.002 ± 0.8233, respectively) and K values (0.891 ± 0.1053 vs. 1.139 ± 0.2264; 1.061 ± 1.2293 vs. 1.395 ± 0.3103, respectively) increased, while the Angle (77.39 ± 2.3917 vs. 73.705 ± 2.1987; 77.084 ± 1.8188 vs. 70.2 ± 2.7268, respectively), MA value (68.877 ± 5.5098 vs. 63.548 ± 4.1688; 68.745 ± 5.6262 vs. 57.716 ± 5.9171, respectively) and CI value (3.75 ± 0.9209 vs. 2.248 ± 0.3944; 3.491 ± 0.7038 vs. 0.841 ± 0.5955, respectively) decreased before and after treatment in the control group and treatment group. Furthermore, differences between R value were not statistically significant (P > 0.05), while differences between K value, Angle, MA value and CI value were statistically significant (P < 0.01) before and after treatment between the two groups, especially in the treatment group.
4.3. The Indexes and Comparison of Mayo Score, UCEIS Score, Geboes Index, Microthrombus and Methylprednisolone in ASUC Patients
Table 2 represents the outcome of Mayo score, UCEIS score and Geboes index in ASUC patients after treatment. The Mayo score, UCEIS score and Geboes index decreased significantly after treatment in both groups (P < 0.01). Furthermore, differences of Mayo score, UCEIS score and Geboes index were statistically significant before and after treatment between the two groups, especially in the treatment group (P < 0.01).
| Control Group | Treatment Group | |
|---|---|---|
| Mayo score (n) | ||
| 1 score | 3 | 9 |
| 2 score | 10 | 22 |
| 3 score | 17 | 8 |
| 4 score | 7 | 4 |
| 5 score | 5 | 1 |
| 6 score | 2 | 0 |
| UCEIS score (n) | ||
| 1 score | 2 | 9 |
| 2 score | 12 | 18 |
| 3 score | 16 | 13 |
| 4 score | 10 | 4 |
| 5 score | 4 | 0 |
| Geboes index (n) | ||
| Level 1 | 0 | 3 |
| Level 2 | 6 | 10 |
| Level 3 | 13 | 17 |
| Level 4 | 18 | 10 |
| Level 5 | 8 | 4 |
The Outcome of Mayo Score, UCEIS Score and Geboes Index in ASUC Patients After Treatment
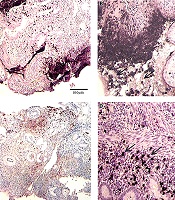
The percentage of microthrombus of ASUC patients in the control group was 38.32% ± 7.73 before treatment and 13.43% ± 3.433 after treatment, while the percentage of microthrombus of ASUC patients in the treatment group was 39.45% ± 8.033 before treatment and 7.68% ± 3.326 after treatment. After treatment, the percentage of microthrombus decreased significantly in both groups (P < 0.01). Furthermore, difference of microthrombus percentages was statistically significant (P < 0.01) before and after treatment between the two groups, especially in the treatment group (Figure 1).
A, before treatment microthrombus immunohistochemical staining by 40 times microscopic examination; B, before treatment microthrombus immunohistochemical staining by 100 times microscopic examination; C, after treatment microthrombus immunohistochemical staining by 40 times microscopic examination; D, after treatment microthrombus immunohistochemical staining by 100 times microscopic examination. The arrows indicate the CD61 positive signal region, indicating the presence of microthrombi.
The dosage of methylprednisolone administered in the control group of ASUC was 1496.82 mg, while the dosage of methylprednisolone administered in the treatment group was 1245.73 mg. The difference in the dosage of methylprednisolone administered between the two groups was statistically significant, and the dosage of methylprednisolone administered in the control group was larger (P < 0.01).
4.4. Adverse Events
No serious adverse reactions, including spontaneous hemorrhage, occurred in the treatment group.
5. Discussion
Studies have revealed that active UC patients exhibit hypercoagulability and have 2 - 4 times greater risk of developing deep venous thrombosis (DVT) and pulmonary embolism (PE) than the normal population. Furthermore, the incidence of autopsy can reach up to 41% (22-24). The results of the present study demonstrated that the blood of ASUC patients was in a hypercoagulable state when compared to patients exhibiting colonic polyps. After anticoagulation treatment, the R and K values significantly increased and platelet count, FIB, D-dimer, Angle, MA and CI value significantly decreased (P < 0.01). Hypercoagulability was also determined to significantly improve. van Bodegraven et al. (25) revealed that inflammatory markers, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and platelet count, and hemostatic parameters (including thrombin-antithrombin complex, FIB and FIB degradation products) were higher in patients with active UC than in healthy controls. After treatment, CRP, ESR, platelet count and FIB values significantly decreased. Shen et al. (26) also reached a similar conclusion.
The current study compared the application value of routine coagulation monitoring and TEG. Compared to TEG, routine coagulation monitoring is often unable to truly reflect the balance of coagulation in patients, and may only describe the process of coagulation in fragments, that is the results are easily affected by anticoagulants such as heparin. The TEG is a curve that dynamically analyzes the whole process of coagulation and FIB formation, and can reflect the interaction between platelets, coagulation factors, FIB, fibrinolytic systems and other cell components (27, 28). The TEG detection has various advantages, including simple operation, short measurement time and permanent retention, which can objectively reflect the changes in real-time coagulation function of patients. The TEG also makes up for the deficiency of conventional coagulation detection items. In foreign countries, TEG has long been used to detect the coagulation function of post-traumatic or perioperative patients and to guide the use of anticoagulant drugs (29, 30). However, the evaluation of TEG in the diagnosis and treatment of UC has been rarely reported in the literature. In the present study, TEG was revealed to quickly detect the overall coagulation function of patients with ASUC, and it was more sensitive than routine coagulation monitoring.
The current study showed that microthrombus formation caused by hypercoagulability in the intestinal mucosa was important to the pathogenesis of UC and formed a pathophysiological basis for anticoagulation therapy. Rivaroxaban that was utilized in the present study is a novel oral anticoagulant primarily used for the treatment of PE and DVT, prevention of joint replacement and systemic thromboembolism. The Mayo score, UCEIS score, Geboes index and the percentage of microthrombus decreased significantly after treatment. The decrease was demonstrated to be significant in the rivaroxaban treated group (P < 0.01). Additionally, the use of rivaroxaban reduced the required dosage of methylprednisolone (P < 0.01) and did not produce any serious adverse reactions. Gul Utku et al. (31) studied the efficacy of rivaroxaban on colitis induced by trinitrobenzene sulfonic acid (TNBS) in 24 rats. The results demonstrated that rivaroxaban exerted a similar effect to methylprednisolone. Rivaroxaban attenuated the accumulation of malonyldialdehyde (MDA) and transforming growth-factor β1 (TGF-β1) and the activites of myeloperoxidase (MPO), matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1. Methylprednisolone reduced only the activity of MPO and the accumulation of MDA and TGF-β1.
Previous studies also revealed that the earlier the mucosal repair begins, the better the clinical prognosis will be (32, 33). To better evaluate the efficacy of rivaroxaban, the Mayo score was used in the present study to evaluate disease severity. Furthermore, endoscopic inflammation of the mucosa was assessed using the UCEIS score. The healing of mucosal tissue was assessed via the Geboes index. The Mayo score, which has been approved by the U.S. Food and Drug Administration, bears information about how doctors can treat the disease and is considered to be the most reliable and effective clinical outcome score for UC. The UCEIS score better unifies the endoscopic diagnosis obtained using different endoscopes and exhibits a high validity, detailed descriptions and good repeatability. The Geboes index is an ideal histological scoring system for UC, which combined application of the endoscopic index can make the evaluation of efficacy more accurate. The results of the present study indicated that rivaroxaban effectively improved the prethrombotic status and intestinal microcirculation of ASUC patients when added to routine treatment. However, there is no effective method to evaluate anticoagulation following rivaroxaban administration. Several studies (34, 35) have reported that TEG can guide rivaroxaban anticoagulation therapy. In the current study, TEG was also used to test the anticoagulation effect. Rivaroxaban discontinued if TEG indicated hypocoagulation.
In conclusion, the blood hypercoagulability of ASUC patients and the presence of microthrombus in intestinal mucosa were demonstrated in this study. Accordingly, routine therapy combined with rivaroxaban can effectively alleviate the clinical symptoms of patients, and may even achieve endoscopic mucosal healing. However, the present study was limited to a small cohort with short follow-up times. Therefore, it is necessary to further assess the potential underlying molecular biological mechanisms and the effects of anticoagulation therapy on the prevention of UC recurrence and so on.