1. Background
Nonalcoholic fatty liver disease (NAFLD) is characterized by hepatic lipid accumulation in hepatocytes without a history of alcohol consumption (1). It is the most common liver disease worldwide with the prevalence of 25-35% in different populations (2). The total prevalence of NAFLD and also the prevalence of mild, moderate and severe fatty liver disease were estimated 33.9% (95% CI: 26.4% - 41.5%), 26.7% (95% CI: 21.7% - 31.7%), 7.6% (95% CI: 5.7% - 9.4%), and 0.5% (95% CI: 0.1% - 0.9%), respectively (3, 4). People with the age range of 40 - 60 years or in some cases younger, are more likely to develop NAFLD.
Epidemiological studies have demonstrated that regardless of the interferer factors, such as obesity and insulin resistance (IR), there is an association between hypovitaminosis D and the presence of NAFLD and steatohepatitis (NASH) (5-7). Furthermore, anti-fibrotic, anti-inflammatory and insulin-sensitizing effects of vitamin D on hepatocytes have been confirmed by several studies (8). Accordingly, it seems that vitamin D possibly plays a critical important role in the pathogenesis of NAFLD (1). It has been shown that the reduced level of 25-hydroxy vitamin D (25(OH)D) is associated with the progression of NAFLD (2, 9-11). In some studies, however, no association was found between vitamin D levels in patients with non-cirrhotic livers and those with liver cirrhosis or also between vitamin D levels in patients with NAFLD and healthy individuals (12). Nevertheless, previous studies have faced some limitations, including a small sample size and no assessment for major interferer factors, such as obesity, insulin resistance, physical activity and energy intake. A recently published study in Asia shows that the association between vitamin D level and NAFLD is varied among the Asian and non-Asian populations (13), which can be due to the differences in the vitamin D receptor alleles among the Asian and Western societies. The results of a recently published systematic review on 5,000 patients with NAFLD and 8,000 healthy individuals (control) demonstrated that there is a vitamin D deficiency in NAFLD patients, indicating that this vitamin could be a part of the pathogenesis of NAFLD. NAFLD patients are 26% more likely to have vitamin D deficiency. This relationship is more obvious by comparing the Western and Eastern societies (14). The increased number of new cases of NAFLD in Iran indicates the importance of raising public awareness and also developing appropriate preventive and therapeutic strategies for management of NAFLD.
2. Objectives
Due to the high prevalence of NAFLD, inconsistent results of different relevant studies and also the effects of environmental and nutritional factors on the pathogenesis of the disease, this study aimed at evaluating the prevalence of vitamin D deficiency in patients with NAFLD and healthy individuals.
3. Methods
This study is a part of a larger project to investigate the factors affecting the development of nonalcoholic fatty liver in an Iranian sample population (Tehran). For this purpose, the information of 2,160 people who referred to a university affiliated nutrition consolidation center following a recall from the early April 2016 to the late September 2017 (a period of little over a year) was randomly selected and investigated. The inclusion criteria included alcohol consumption less than 30 g/d for men and 20 g/d for women and the subjects’ willingness to participate in the study (15). Exclusion criteria also included those with diabetes, cancer, hypothyroidism or other disorders or taking medications that influence the metabolic status of the body. In addition, those who changed their diet over the last six months and consumed the supplements containing vitamin D, were also excluded.
The study protocol was in accordance with the Helsinki Declaration and confirmed by the Ethics Committee of Fasa University of Medical Sciences (approval code: IR.FUMS.REC.1396.267). The participants were informed about the research objectives and the written informed consent was obtained from the subjects before starting the survey. The study was supported by the Fasa University of Medical Sciences (grant No.: 96050).
The demographic characteristics, including age, gender and woman’s menopausal status were asked from the participants. The weight and height of the subjects were measured and recorded by a trained researcher using a stadiometer accurate to 0.1 cm and the Seca 767 digital scale (± 0.1 kg; Seca; Japan), respectively. Body mass index (BMI) was also calculated as weight in kilograms divided by height in meters squared.
Blood pressure was measured 15 minutes after rest in the sitting position two times within a 15-minute interval from the right arm of the subjects. The mean value of these two measurements was considered as the final blood pressure.
The subjects’ food intake data over the past year were collected by the semi-quantitative food frequency questionnaire (FFQ). FFQ includes a list of 168 food items and also portion size information as standardized portions. The subjects were asked if they had eaten a particular food item (standardized portions) within the last year. The collected values for each food were converted into daily intake (g/day) using a household scales guidebook, based on which the energy intake of the subjects was calculated.
To measure the levels of serum lipids, blood sugar and insulin, 10 cc venous blood sample was drawn after 12 hours of fasting. The blood sample was then transferred to the laboratory under appropriate conditions to perform biochemical tests. High-density lipoprotein (HDL), triglyceride (TG) and total cholesterol levels were measured using enzymatic method by commercially available kits (Pars Azmoon Laboratories, Iran). In addition, the Friedewald formula (FF) was employed to calculate low-density lipoprotein (LDL) level (16). Eventually, the total values of serum lipids were reported as mg/dl. Using 75 g glucose, we measured 2-hour plasma glucose and insulin.
Homeostatic model assessment (HOMA) is used for assessing IR and β-cell function (7).
25(OH)D was measured through the enzyme-linked immunosorbent assay (ELISA) kits (item No.: 501050; Cayman Chemical Co., USA). Its levels were then classified based on the results of Lips study (17). The values below 12.5 nmol/L, between 12.5 and 25 nmol/L, between 25 and 50 nmol/L and higher than 50 nmol/L were considered as severe vitamin D deficiency, moderate vitamin D deficiency, mild vitamin D deficiency and vitamin D replete, respectively.
Fatty liver index (FLI) was used to measure liver steatosis (18). The participants were assigned into three groups based on the FLI values: FLI < 30 was defined as not having NAFLD, FLI ≥ 60 was defined as having NAFLD and patients with FLI of 30 - 59 were defined as having intermediate FLI. The results were then confirmed (Pearson correlation = 0.698, P value < 0.001) by an Gastroenterologist with the extent of stiffness of liver mass using controlled attenuation parameter (CAP) measured with FibroScan XL probe, which is a more reliable diagnostic tool for the obese people and those who have high fat levels (19-21).
FLI = [e0.953 × loge (TG) + 0.139 × BMI + 0.718 × loge (GGT) + 0.053 × waist circumference - 15.745)]/[1 + e0.953 × loge (TG) + 0.139 × BMI + 0.718 × loge (GGT) + 0.053 × waist circumference - 15.745] × 100, with triglycerides measured in mmol/l, GGT in U/L, and waist circumference in cm.
3.1. Statistical Methods
The data were considered to be normally distributed. Based on the normal distribution results, for descriptive statistics, the mean ± SD and No. (%) were used for quantitative and qualitative variables, respectively. Univariate analysis was performed using the chi-Square test or independent samples t-test. To assess the simultaneous effect of variables on NAFLD, logistic regression was implemented and the linear regression was also applied to find a model based on effective factors on NAFLD. For logistic regression, we proceeded in two steps; initially, only the vitamin D variable was entered. In the next step, all related variables were analyzed by Forward: conditional method and the amount of variance explained in each step was investigated. Two model was selected. The first model with three variables and the second model with four variables explained 82.5% and 82.5% of the variance, respectively. Accordingly, the second model with three variables was selected as the optimal model. To assess the model accuracy, the probability of NAFLD for each participant was estimated using the proposed model and the optimal cut-off point was obtained using the receiver operating characteristic (ROC) curve analysis. All the statistical analyses were performed by MedCalc 14.0 (Medcalc software, Ostend, Belgium) or SPSS 20.0 (IBM Co., Armonk, NY, USA).
4. Results
2,160 subjects participated in the study, of whom 745 subjects (34.5%) had NAFLD, whereas 1,415 subjects (65.5%) were healthy. The demographic characteristics of the subjects based on the groups are shown in Table 1. Almost all studied variables, except for the gender and smoking showed a significant difference between two groups. The odds ratio values in the univariate model were calculated for all factors associated with fatty liver. It can be stated that all variables were found to increase the risk of NAFLD.
| Healthy People (N = 1415) | NAFLD Patient (N = 745) | P Value | OR | 95% CI | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age, y | 35 ± 9 | 49 ± 8 | < 0.001 | 1.19 | 1.173 | 1.207 |
| Weight, kg | 80.88 ± 12.27 | 96.16 ± 11.50 | < 0.001 | 1.103 | 1.093 | 1.113 |
| BMI, kg/m² | 25.43 ± 4.10 | 30.95 ± 3.49 | < 0.001 | 1.413 | 1.371 | 1.457 |
| Waist circumference, cm | 96.49 ± 16.04 | 118.10 ± 12.77 | < 0.001 | 1.097 | 1.088 | 1.106 |
| Physical activity, MET/24h | 24.9 ± 5.0 | 18.3 ± 4.5 | < 0.001 | 0.762 | 0.744 | 0.781 |
| Energy, kcal | 2405.49 ± 413.73 | 2664.98 ± 460.11 | < 0.001 | 1.001 | 1.001 | 1.002 |
| FBS, mg/dL | 99.39 ± 13.53 | 118.29 ± 13.15 | < 0.001 | 1.102 | 1.092 | 1.111 |
| Fast Insulin, µU/mL | 8.16 ± 3.78 | 14.17 ± 3.39 | < 0.001 | 1.496 | 1.446 | 1.547 |
| Two hour glucose, mg/dL | 115.22 ± 19.05 | 130.51 ± 20.18 | < 0.001 | 1.039 | 1.034 | 1.044 |
| Two hour insulin, µU/mL | 40.28 ± 12.44 | 54.67 ± 12.40 | < 0.001 | 1.093 | 1.084 | 1.103 |
| HOMA-IR | 0.92 ± 0.24 | 1.17 ± 0.23 | < 0.001 | 70.605 | 45.294 | 110.060 |
| HOMA-B | 68.18 ± 15.90 | 79.28 ± 18.02 | < 0.001 | 1.040 | 1.034 | 1.046 |
| LDL, mg/dL | 93.72 ± 15.56 | 113.71 ± 16.61 | < 0.001 | 1.077 | 1.069 | 1.085 |
| HDL, mg/dL | 48.18 ± 7.95 | 38.88 ± 7.08 | < 0.001 | 0.858 | 0.846 | 0.871 |
| TG, mg/dL | 186.57 ± 26.15 | 214.28 ± 25.03 | < 0.001 | 1.041 | 1.037 | 1.045 |
| Total cholesterol, mg/dL | 176.01 ± 17.11 | 195.40 ± 16.18 | < 0.001 | 1.072 | 1.064 | 1.079 |
| Systolic blood pressure, mmHg | 12.47 ± 1.63 | 13.09 ± 1.66 | < 0.001 | 1.256 | 1.188 | 1.327 |
| Diastolic blood pressure, mmHg | 8.04 ± 1.02 | 8.84 ± 0.96 | < 0.001 | 2.205 | 1.997 | 2.435 |
| ALT, IU/L | 39.77 ± 13.65 | 59.38 ± 13.50 | < 0.001 | 1.104 | 1.094 | 1.113 |
| AST, IU/L | 32.40 ± 13.87 | 49.51 ± 12.62 | < 0.001 | 1.094 | 1.085 | 1.103 |
| GGT, IU/L | 23.64 ± 10.14 | 36.86 ± 12.98 | < 0.001 | 1.102 | 1.092 | 1.113 |
| Vitamin D, nmol/L | 26.77 ± 8.26 | 15.84 ± 5.50 | < 0.001 | 0.804 | 0.789 | 0.820 |
| Gender | 0.001 | 0.741 | 0.621 | 0.886 | ||
| Female | 656 (62.1) | 401 (37.9) | ||||
| Smoking | 0.914 | 1.013 | 0.799 | 1.284 | ||
| Yes | 240 (17) | 125 (16.8) | ||||
Demographic and Basic Information of the Participants in Healthy and Patient Groupsa
In the studied subjects, no participant had adequate levels of vitamin D and 845, 1,055, and 260 subjects had mild, moderate, and severe vitamin D deficiency, respectively.
Using regression model, we tried to determine the role of vitamin D in the development of NAFLD. For this purpose, different variables (age, sex, woman’s menopausal status, BMI, waist circumference, waist-to-hip ratio (WHR), waist-to-height ratio (WHtR), physical activity, fasting blood sugar (FBS), 2 hours blood glucose, fasting insulin, 2 hours insulin, HOMA-IR, LDL, HDL, total cholesterol, TG, systolic blood pressure, diastolic blood pressure, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), total energy intake and vitamin D) were introduced into a stepwise model and the level of inferential variance was examined by the models. Three regression models were chosen. In the first model, only vitamin D was introduced. In this model, by considering the mild vitamin D deficiency as the reference, the risk of NAFLD for the groups with moderate and severe vitamin D deficiencies obtained about 5 and 13, respectively. In the second model, in addition to vitamin D, age and fasting insulin variables were also introduced and the model explained about 82.5% of the variance. Those with moderate and severe vitamin D deficiencies were 4.2 and 2.4 times more at risk for developing NAFLD, respectively. In the third model, ALT was added and the model explained 82.5% of the variance. It was found that those with moderate and severe vitamin D deficiencies were 3.6 and 2.1 times more at risk for developing NAFLD compared to the mild deficiency (Table 2).
| Model 1aOR (95% CI) | Model 2bOR (95% CI) | Model 3cOR (95% CI) | |
|---|---|---|---|
| Age, y | - | 1.144 (1.114 - 1.175) | 1.079 (1.058 - 1.099) |
| Fasting insulin, µU/mL | - | 1.284 (1.189 - 1.386) | 1.183 (1.131 - 1.238) |
| ALT | 1.029 (1.018 - 1.040) | ||
| Vitamin D mild deficiency | Ref | Ref | Ref |
| Vitamin D moderate deficiency | 5.858 (4.144 - 8.281) | 4.257 (2.498 - 7.255) | 3.616 (2.104 - 6.214) |
| Vitamin D severe deficiency | 13.914 (10.149 - 19.075) | 2.416 (1.669 - 3.497) | 2.148 (1.476 - 3.126) |
Relationship Between Vitamin D Levels and the State of Disease
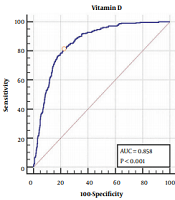
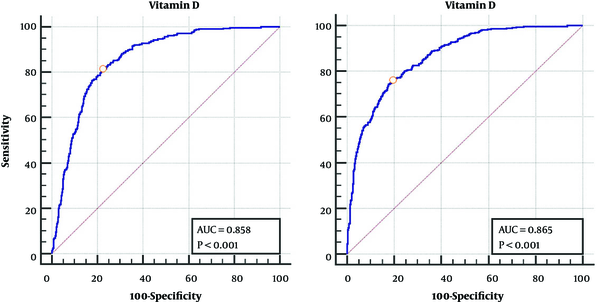
Table 3 shows the cut-off point for vitamin D in men and women, vitamin D levels lower than 18 (nmol/L) for women and 21 (nmol/L) for men significantly increase the risk of NAFLD (Figure 1).
| Cut Point | Sensitivity | Specificity | AUC | PPV | NPV | PLR | NLR | |
|---|---|---|---|---|---|---|---|---|
| Female | 18.25 | 76.06 | 80.64 | 86.5 | 66.93 | 86.73 | 3.90 | 0.30 |
| Male | 21.5 | 81.40 | 77.73 | 85.8 | 65.31 | 89.02 | 3.66 | 0.24 |
Receiver Operating Characteristic Curve Analysis for Defining the Ideal Vitamin D Cut-Off Point Based on Nonalcoholic Fatty Liver Diseasea
5. Discussion
The results showed an association between 25(OH)D and NAFLD independent variables, including anthropometric indicators, energy intake, physical activity and biochemical indices. Furthermore, the developed models for predicting NAFLD based on the various variables suggested a significant correlation between NAFLD and age, fasting insulin and the level of 25(OH)D. In addition, our study indicated that 20 - 25 nmol/L of 25(OH)D is an acceptable level to prevent NAFLD in men and women.
A recently published systematic review including 45 cross-sectional studies, revealed that 29 studies (64.4%) reported an inverse relationship between vitamin D levels and NAFLD, whereas 16 studies (35.6%) were unable to demonstrate a correlation between these two (22). The optimal threshold for vitamin D in chronic liver disease has reported 10 to 32 ng/mL in (22), which is equal to 24 - 80 nmol/L.
NAFLD is a multifaceted condition, affecting various aspects of the patient’s life, in which several factors, including genetics (23), lifestyle (24), diet (25) and anthropometric factors (26) are involved. Vitamin D has recently been attracted attention as NAFLD risk factor as numerous studies have investigated the association between the levels of serum vitamin D and the risk of NAFLD. Consistent with our results, almost all studies have found that vitamin D deficiency is correlated with the increased risk of NAFLD (2, 6). Bril et al. study on the relationship between NAFLD and serum vitamin D level showed no correlation between these variables, which is not consistent with our results (27). However, since the majority of the relevant studies have not been able to study all affecting variables and modify them, no reliable conclusions have yet been provided. On the other hand, several studies evaluated only a limited number of factors, including ALT to diagnose NAFLD, which reduces the accuracy of their studies.
Various mechanisms have been proposed for the relationship between vitamin D and the incidence of NAFLD. Vitamin D plays its significant intracellular role through bonding with vitamin D receptor (VDR). It has been shown that VDR influences the expression of over 200 genes involved in metabolism, of which inflammation (28) or cellular differentiation (29) should be considered as inflammation plays a significant role in developing NAFLD (30). VDR is also expressed by macrophages. In animal studies, it has been shown that the active form of vitamin D is able to enhance stability of mRNA of the IκB-α (inhibitor of NF-κB) and reduce its phosphorylation, indicating the anti-inflammatory effect of vitamin D in macrophages (31).
The results of genome-wide association studies (GWAS) on patients with NAFLD have shown that the mutation in the vitamin D-binding protein gene (as the main vector of vitamin D) is one of the four major polymorphisms associated with the risk of NAFLD (32). It has been shown that VDR gene expression has an inverse relationship with the intensity of NAFLD (33). On the other hand, it has been shown that vitamin D levels have a positive and significant correlation with the level of adiponectin, independent of BMI (34). Adiponectin as an anti-inflammatory factor has an inverse relationship with NAFLD severity (35).
Our proposed model for the variables affecting the development of NAFLD suggests that age, fasting insulin level and vitamin D status are the strongest factors to predict incidence of NAFLD. In this regard, using the minimum number of variables, we could predict the risk of NAFLD. The area under the ROC curve for this model obtained 0.96 (95% CI: 0.95 - 0.97). Accordingly, we could predict the risk of NAFLD using fewer variables used in relevant studies. Lin (36) announced that BMI, hemoglobin, fasting glucose and triglyceride are the major variables to predict the risk of NAFLD. However, in another study, BMI, WHR, triglyceride, glucose, systolic blood pressure and ALT were reported as the major influential factors in the used model (37). It should be noted that compared to other relevant studies, in our study, no anthropometric variables were entered into the main model. This suggests that metabolic variables, including fasting insulin level possibly play a significant role in enhancing the susceptibility to NAFLD. Anthropometric variables, such as the waist size or BMI may exert their effect through altering the level of metabolic factors, including fasting insulin level (38, 39). Analysis of mediator variables are needed to confirm this finding (26).
According to our results, the cutoff point of vitamin D level to increase the risk of NAFLD was 18.25 and 21.5 nmol/L for women and men, respectively. Using the Youden’s index, which is calculated as the maximum sum of sensitivity and characteristics, the area under the curve for the cutoff point obtained 0.865 and 0.858 for women and men, respectively.
In many studies, the level of vitamin D has been compared in patient and healthy individuals. In a meta-analysis, Eliades et al. (14) investigated the level of vitamin D in patients with NAFLD and concluded that those with NAFLD had 0.36 ng/mL (95% CI: 0.32 - 0.40) lower serum vitamin D level compared to the healthy subjects and also they were 1.26 times more likely to suffer from vitamin D deficiency (OR: 1.26, 95% CI: 1.17 - 1.35). By converting to nmol/L, the difference in vitamin D level between two groups obtained 1 nmol/L. However, the mean difference between the two groups in our study was about 12 nmol/L.
This study faced some limitations. This research was a cross-sectional study, which limited us to draw a causative conclusion. Nevertheless, the studied population was larger than most of other studies. One of the strengths of our study is using volunteer healthy people who had not a history of NAFLD, so as a population-based study, its results can be generalized to the community. Our results showed that an appropriate level of vitamin D can prevent the development of NAFLD. Moreover, the samples were selected by random sampling from those who referred to the medical center following a recall through a year. Although it minimized the possibility of selection bias, however sampling in different seasons was another limitation of this study. Using FibroScan device as a verified method to study NAFLD (18, 40), confirming the results by a gastroenterologist using FibroScan device as well as the large number of factors affecting NAFLD can enhance the reliability of our research. However, conducting a longitudinal study at multicenter level is suggested to obtain more accurate results.
5.1. Conclusions
The results of various studies and also our findings indicate a significant relationship between vitamin D levels and the risk of NAFLD. Particularly, our study suggests that the values less than 18 nmol/L for women and 21 nmol/L for men can increase the risk of NAFLD. Accordingly, the level of 25 nmol/L can be introduced as the threshold for preventing NAFLD in the community. In conclusion, it is important to maintain the appropriate serum level of vitamin D in preventing NAFLD that can be provided by consuming a vitamin D-rich food basket or by more exposure to sunlight.