1. Introduction
Tebuconazole is a triazole fungicide used agriculturally for the treatment of plant pathogenic fungi. Its mechanism of action is to inhibit the biosynthesis of sterol in fungi (1). It has been listed among the top 10 active ingredients in the EU’s fungicide report (2). Tebuconazole is applied to various products, in particular, vegetables and some fruits such as grapes (3).
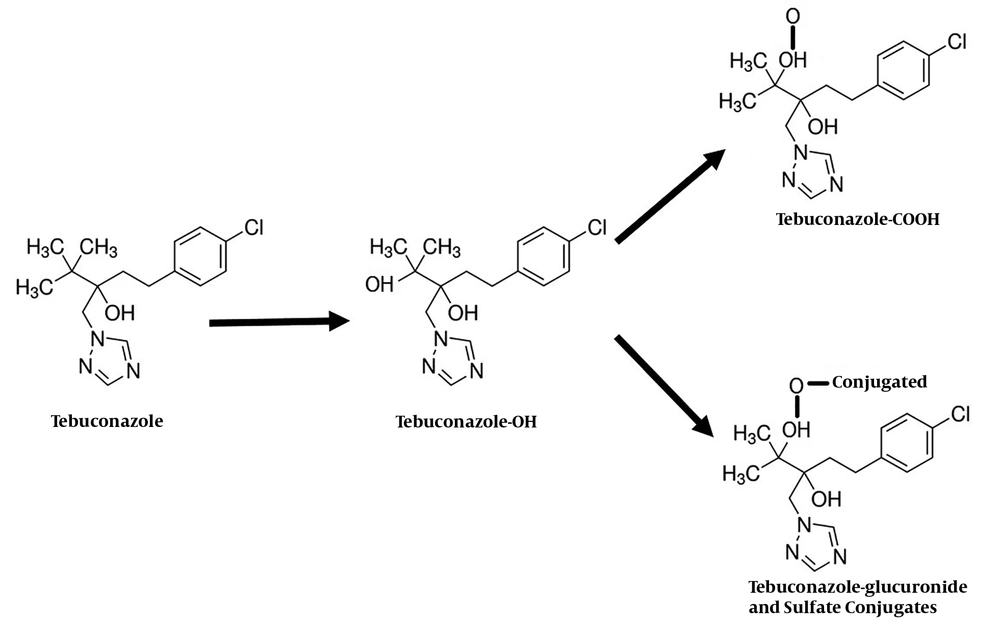
So far, the pathways of tebuconazole biotransformation have not been studied in humans. In animal models, however, the biotransformation involves the sequential oxidation of a methyl group in the butyl chain to yield alcohol and carboxylic acid metabolites Tebuconazole-OH (tebuconazole-1-hydroxy) and Tebuconazole-COOH (tebuconazole carboxylic acid). In the next step, tebuconazole-OH is conjugated to form the glucuronide and sulfate conjugates, which are excreted in the urine. Unchanged tebuconazole is excreted in small amounts via the urine, as well (4). An overview of the tebuconazole metabolic pathway is shown in Figure 1.
Here, we report the case of a 48-year-old male patient with tebuconazole-induced hepatitis from Iran, who was successfully treated merely with exposure avoidance.
2. Case Presentation
According to the requirements of the local ethics committee, written informed consent was obtained for the publication of the patient’s medical information. In May 2018, a 48-year-old male with respiratory complaints including productive cough, anorexia, weakness, weight loss of 4 kg, sweating, and increased levels of liver enzymes from two weeks ago was referred to the clinic of infectious diseases at Imam Khomeini Hospital, Ardabil, Iran. The patient was living in a rural area. The occupational history taking showed that the patient was a farmer currently working on planting barley. He had a history of constant contact with agricultural pesticides. No past history of medical or surgical conditions was reported.
On the physical examination, the patient appeared to be ill. On admission, blood pressure was 125/75 mmHg, the heart rate was 96 beats per minute, the respiratory rate was 22 per minute, and the body temperature was 37.4°C. Chest auscultation revealed generalized expiratory wheezing in both lung fields. There was tenderness to palpation in the right upper quadrant of the abdomen. The liver and spleen were not palpable.
Initial investigations showed increased levels of liver enzymes. The baseline laboratory test results are shown in Table 1. His chest X-ray revealed no abnormality and the sputum smear stains were negative for Mycobacterium tuberculosis. Serological markers for the diagnosis of hepatitis B virus infection such as Anti-HBc (hepatitis B core antibody), HBsAg (hepatitis B surface antigen), HBeAg (hepatitis B envelope antigen), and Anti-HBe (antibody to HBeAg) were non-reactive. Tests to detect anti-hepatitis E virus (HEV) and anti-hepatitis A virus (HAV) were both negative for immunoglobulin M (IgM). A test to detect hepatitis C antibody (anti-HCV) was negative. Based on the findings, acute and chronic viral hepatitis was ruled out. Antibodies for the detection of human immunodeficiency viruses type-1 and type-2 (HIV-1 and HIV-2) were negative. Tests to detect antibodies to Epstein-Barr virus (EBV) and cytomegalovirus (CMV) were both negative for IgM. In the next step, iron profile, serum ceruloplasmin, alpha-1 antitrypsin, and autoimmune markers were also evaluated, showing no evidence to confirm the diagnosis of hemochromatosis, Wilson disease, alpha-1 antitrypsin deficiency, and autoimmune hepatitis, respectively.
| Test | On Admission | Two Weeks After Avoiding Tebuconazole | Normal Ranges |
|---|---|---|---|
| White blood cell (WBC), per mm3 | 6720 | 8200 | 4500 to 10000 |
| Hemoglobin (Hb), g/dL | 12.8 | 14.1 | Male: 13.5 to 17.5; female: 12.0 to 15.5 |
| Platelets count, per μL | 202000 | 358000 | 150000 to 450000 |
| Erythrocyte sedimentation rate (ESR), mm/h | 38 | 21 | 0 to 22 |
| Aspartate aminotransferase (AST), IU/L | 125 | 13 | Males: 6 to 34; females: 8 to 40 |
| Alanine aminotransferase (ALT), IU/L | 220 | 17 | 7 to 56 |
| Alkaline phosphatase (ALP), IU/L | 511 | 240 | 44 to 147 |
| Total bilirubin, mg/dL | 1.5 | 1.3 | 0.31 to 1.94 |
| Serum creatinine, mg/dL | 1.1 | 0.8 | 0.84 to 1.21 |
In more detailed history taking, the patient declared the use of a fungicide called tebuconazole to blend with barley seeds before planting while, unlike his colleagues, he was not using masks and gloves. In addition, the patient stated that pruritus, redness of the eyes, and mild cough appeared after the completion of the work. Therefore, his medical history, the physical examination, and laboratory tests indicated tebuconazole poisoning. The patient was discharged and advised to avoid any exposure to the fungicide. Two weeks after discharge, we rechecked the patient’s tests. Interestingly, the results of the new tests showed complete improvement in the levels of liver enzymes.
3. Discussion
The use of fungicidal agents is very common in agriculture although environmental contamination is a concern. To prevent the contaminants, it is essential to manage the use of fungicidal agents. Tebuconazole is a systemic fungicidal agent from the triazole group, which prevents the biosynthesis of ergosterol in the fungal cell membrane and stops the growth of fungal pathogens (5). Tebuconazole is available with two brand names, Roxil and Folicur, to use in planting seeds, especially wheat and barley, against fungal diseases such as hidden and obvious blackheads (6). This fungicide is marketed in the form of colorless crystals that are soluble in most organic solvents. Tebuconazole has good resistance in soil and seed and is resistant to high temperature, as well. This compound is harmful to bees, fish, and other creatures (7).
Triazole Conazole fungicides are metabolized in liver microsomes (8). Many pesticides and poisons accumulate in high concentrations in the liver. As a result, the most destructive effects of toxins are visible in this organ (9). In the present study, as reported, two weeks after avoiding the poison, the liver enzymes returned to their normal state and the symptoms disappeared. No study has been done so far to explore the human pathogenesis of this poison, but several studies have been conducted on its pathogenicity in other living organisms. In order to assess the toxic effect of tebuconazole by histological tissue examination of gills and liver of Labeo rohita, Selvi et al. studied liver and tissues exposed to a lethal concentration of tebuconazole, and reported extensive vacuoles and hepatocytes necrosis and hepatic capillary congestion (10).
In addition, Knebel et al. evaluated the toxic effects of propiconazole and tebuconazole on the liver. Their results showed that tebuconazole acted as an antagonist of the constitutive androstane receptor, whereas propiconazole acted as an agonist of this receptor (11).
In conclusion, the risk of developing hepatitis when being exposed to tebuconazole should be considered. Protective measures and necessary precautions during handling should be taught to farmers, including wearing gloves and masks and working in ambient air.