1. Background
Hepatitis B virus (HBV) infection is a major health burden that affects people globally. There are approximately 240 million people chronically infected with HBV (1). The HBV infection results in a severely heightened risk of developing cirrhosis and hepatocellular carcinoma (HCC) (2-4).
Hepatocellular carcinoma ranks third concerning cancer-related mortality, demonstrating a rising tendency, and is the fifth most frequently diagnosed malignancy in the world (5, 6). Routine surveillance is offered to patients with cirrhosis of the liver or advanced chronic liver disease with the aim of detecting HCC at potentially curable stages (7-11).
It remains challenging to identify patients with chronic hepatitis B (CHB) with a high HCC risk independent of risk factors such as cirrhosis (12, 13), hepatitis C virus (HCV) coinfection (14, 15), hepatitis D virus (HDV), or substance abuse (16). Effective, routinely applicable predictive tools are currently lacking to improve the identification of the individual CHB patient’s risk for HCC development.
The FIB-4 index was originally intended to be a tool to noninvasively predict liver fibrosis and cirrhosis in patients co-infected with human immunodeficiency virus (HIV)/HCV (17). In CHB (18-20) and nonalcoholic fatty liver disease (NAFLD) (21), this score demonstrated moderate to high accuracy for the detection of advanced fibrosis.
The possible role of FIB-4 in predicting HCCs in Korean hepatitis B surface antigen (HBsAg) carriers was investigated by Suh et al. In this population, elevated FIB-4 values were a good predictor for HCC incidence (22). They proposed a FIB-4 value of < 1.25 as a cutoff for a low risk. In a recent publication, Demir et al. (23) showed that a FIB-4 value above the proposed cutoff ≥ 1.25 is an unreliable clinical indicator for HCC development in European, predominantly non-Asian patients with CHB.
2. Objectives
This study aimed to validate the modified cutoff values in a predominantly Caucasian CHB population from a university hospital outpatient liver unit in Germany to instate FIB-4 as a possible tool in stratifying the risk of HCC development.
3. Methods
We evaluated 655 hospital records of CHB patients over the age of 18. They had been referred to our outpatient liver unit at the University Hospital of Cologne, Germany, during a timeframe between January 1994 and June 2011. Complying with German law, approval of our local ethics committee or written informed consent from participants was not required in retrospective studies (paragraph 15, sentence 1, Nordrhein Medical Association’s professional code of conduct from 14 November 1998 as amended on 19 November 2011 / paragraph 6, sentence 1, Health Data Protection Act of Nordrhein-Westfalen).
A prerequisite for study inclusion was at least three CHB-related visits within 24 months during our study timeframe. Hepatitis B was considered chronic if HBsAg and/or HBV DNA > 10 IU/mL were detectable over a period of at least six months. Patients who had only two visits were eligible for inclusion if their period of surveillance lasted a minimum of one year and their status regarding survival (with or without HCC development) until April 2015 was known. Overall, 305 patients did not meet our inclusion criteria, which resulted in an exclusion from our analysis: 13 patients because of HCC diagnosis at their first visit, 51 patients because of a coinfection with HDV (n = 16), HCV (n = 22), and HIV (n = 13), and 219 patients due to only one or two visits. Additionally, 23 patients of Asian ethnicity were excluded from the analysis. Thus, a total of 350 CHB patients remained eligible for inclusion. Survival and HCC data for all included patients were updated by the reference date April 30th, 2015. Overall, 23 patients were lost to follow up and their survival status (with or without HCC) as of April 30th, 2015 remained unknown. For these cases, the last known status was used in the analysis. The primary endpoint of our analysis was HCC development during our observational period in relation to FIB-4 levels.
We recorded patients’ demographics, various HBV markers (i.e. quantitative HBV DNA, HBsAg, and hepatitis B envelope antigen (HBeAg)), body weight and height, alpha-fetoprotein (AFP), hepatic panel, prothrombin time, platelet count, alcohol consumption, and the results from liver imaging in each visit. We also assessed the type and duration of antiviral treatment and analyzed liver histology by employing the Desmet score (24). Cirrhosis and fatty liver (FL) were diagnosed based on either histology or imaging. We carried out ultrasound, CT scans, or MRI in clinical routine. The individual HCCs were diagnosed in accordance with valid international recommendations for the respective time points of diagnosis (25-28). During our study timeframe from 1994 to 2015, these methods included liver biopsy, ultrasound, Contrast-enhanced Ultrasound (CEUS), contrast-enhanced CT, MRI, and AFP measurements. Biopsies of suspected nodules were usually performed only when the diagnosis based on imaging/AFP was unclear.
The analysis of the population’s baseline characteristics was carried out using descriptive statistics. We used the data from the baseline visit to calculate the FIB-4 index (age [years] × aspartate aminotransferase [U/L]/(platelet counts [109/L × alanine aminotransferase [U/L1/2) (17) for each patient.
We divided patients into two groups based on their FIB-4 values, using a cutoff of < 0.3635 (FIB-4reference) representing the 50th percentile value for a low risk versus a cutoff of ≥ 0.3635 for an elevated risk (FIB-4elevated). Since in routine clinical practice, all patients with an elevated risk would be offered a strict form of surveillance, we opted against a division into further subgroups with moderate, high, and very high risk, respectively. We also analyzed the study population’s baseline characteristics according to the respective FIB-4 groups. In the development of HCC in CHB patients, liver cirrhosis remains the main risk factor. To determine the validity of our cutoff values in patients without cirrhosis, we performed an additional complete sub-analysis (including baseline characteristics, Kaplan-Meier analysis, and Cox regression), exempting patients with liver cirrhosis upon the first presentation.
To analyze the association of FIB-4 ≥ 0.3635 with HCC, we employed Cox’s proportional hazards models, adjusted for age, sex, amount of alcohol consumption, type 2 diabetes, body mass index (BMI), antiviral medication at baseline (or during observation), and duration of CHB infection.
We analyzed the data with SPSS version 25 (SPSS, IBM Inc., Chicago, IL). We expressed numeric variables as means ± standard deviations (SD) or medians (ranges) and compared them using the Mann-Whitney U test. The cumulative HCC incidence during the surveillance period was calculated using the Kaplan-Meier method. Categorical variables were expressed as numbers and percentages and compared using the chi-square test. For all calculations, a P value of < 0.05 was considered statistically significant.
4. Results
Our study population’s baseline characteristics are detailed in Table 1. At baseline, the mean age of patients was 41 years (± 13.6 years) and 229 (65%) patients were of the male gender. The median duration of follow-up totaled 107 months with a range of 12 - 255 months and 23 (7%) patients were lost to follow up.
| CHB without HCC During Observation (N = 327) | CHB with HCC During Observation (N = 23) | P Value | |
|---|---|---|---|
| Mean age ± SD, y | 40 ± 13.1 | 57 ± 9.0 | < 0.001 |
| Male gender, No. (%) | 210 (64) | 19 (83) | 0.086 |
| Documented ethnicity, No. (%) | 319 (98) | 23 (100) | |
| Northern Europe | 104 (32) | 3 (13) | |
| Southern Europe/Middle East/North Africa | 159 (49) | 12 (52) | 0.056 |
| Eastern Europe/former USSR | 35 (11) | 7 (30) | 0.377 |
| Sub-Saharan Africa | 21 (6) | 1 (4) | |
| Duration of chronic hepatitis B infection, months | 76 (12 - 497) | 166 (12 - 514) | 0.001 |
| Hepatitis B surface antigen (HBsAg), No. (%) | 327 (100) | 23 (100) | |
| Hepatitis B envelope antigen (HBeAg) positive, No. (%) | 65 (20) | 3 (13) | 0.309 |
| Quantitative HBV DNA, IU/mL | 3540 (< 10 - 295,000,000) | 30 (< 10 - 9,880,000) | 0.068 |
| Antiviral therapy at baseline, No. (%) | 49 (15) | 7 (30) | 0.072 |
| Antiviral therapy during observation, No. (%) | 187 (57) | 18 (78) | 0.051 |
| (Pegylated) Interferon | 11 (3.5) | 1 (4) | |
| Lamivudine 100 mg | 31 (9.5) | 6 (28) | 0.02 |
| Entecavir 0.5/1 mg | 10 (3) | 0 (0) | |
| Tenofovir 245 mg | 7 (2) | 0 (0) | |
| Laboratory parameters | |||
| Albumin [35 - 52 g/L] | 43 (23 - 70) | 38 (27 - 45) | < 0.001 |
| Aspartate aminotransferase (AST) [< 40 U/L] | 35 (6 - 1387) | 49 (17 - 153) | 0.013 |
| Alanine aminotransferase (ALT) [< 50 U/L] | 46 (5 - 1834) | 70 (16 - 239) | 0.045 |
| Gamma-glutamyl-transferase (GGT) [< 60 U/L] | 29 (3 - 1714) | 82 (29 - 469) | < 0.001 |
| Total bilirubin [< 1.1 mg/dL] | 0.6 (0.2 - 18.7) | 1.1 (0.3 - 2.5) | 0.008 |
| Platelet counts [150 - 400 ×109/L] | 206 (29 - 478) | 125 (65 - 387) | < 0.001 |
| Prothrombin time [70 - 120 %] | 97 (10 - 135) | 79 (52 - 112) | 0.001 |
| Alpha fetoprotein (AFP) [< 5.7 kU/L] | 3 (0.8 - 156) | 35 (2 - 2030) | < 0.001 |
| Liver cirrhosis, No. (%) | 30 (9) | 11 (48) | 0.001 |
| MELD score | 7 (6 - 22) | 9 (6 - 15) | 0.274 |
| Fatty liver on imaging, No. (%) | 104 (32) | 11 (48) | 0.069 |
| BMI | 25.5 (16.8 - 40.6) | 26.9 (20.7 - 35.0) | 0.554 |
| Type 2 diabetes, No. (%) | 22 (7) | 4 (17) | 0.095 |
| Arterial hypertension, No. (%) | 49 (15) | 9 (39) | 0.004 |
| Alcohol consumption, g/d | 31 (0 - 200) | 40 (12 - 60) | 0.573 |
| Follow-up, mo | 109 (12 - 255) | 39 (14 - 211) | 0.002 |
| Number of presentations | 8 (2 - 62) | 12 (3 - 46) | 0.156 |
| Follow-up-interval, months | 12 (1 - 93) | 4.2 (0.9 - 11) | < 0.001 |
| Lost to follow up at end of study, No. (%) | 23 (7) | 0 (0) | |
| FIB-4 values | 0.35 (0.06 - 7.81) | 0.81 (0.10 - 3.76) | < 0.001 |
Baseline Characteristics of the Study Population (N = 350)a
Using origin rather than nationality as a basis, we analyzed a diverse population of non-Asian descent. The vast majority of our patients originated from the Middle East, southern Europe, and North Africa.
Comparing CHB patients with and without HCC during observation, certain statistically significant differences arose. Patients who developed HCC had a longer duration of CHB infection (median 166 months, range 12 - 514 months, P = 0.001), a higher age (57 ± 9.0 years, P = 0.001), a higher rate of liver cirrhosis (48%, P = 0.001), arterial hypertension (39%, P = 0.004), and more abnormalities in laboratory parameters (P ≤ 0.025) at baseline. Proportionally, more HCC patients received lamivudine treatment (28%, P = 0.02). These patients showed a tendency towards lower levels of HBV DNA (median 30 IU/mL, range < 10 - 9,880,000 IU/mL, P = 0.068 n.s.). Comparing patients with HCC during observation to those without HCC, the median FIB-4 value was significantly higher in the HCC group [0.81 (0.10 - 3.76) versus 0.35 (0.06 - 7.81; P < 0.001)] (Table 1).
The baseline characteristics according to FIB-4 groups are shown in Table 2. As shown, 174 patients (106 males) were in the FIB-4elevated bracket (≥ 0.3635). Of these, 21 (12%) developed HCC during observation compared to only two patients (1%) in the FIB-4reference (< 0.3635) group. Only 3% of patients with liver cirrhosis had low FIB-4 levels (< 0.3635).
| FIB-4, < 0.3635 (N = 176) | FIB-4, ≥ 0.3635 (N = 174) | Total (N = 350) | |
|---|---|---|---|
| Age in years, No. (%) | |||
| 18 - 39 | 95 (54) | 15 (9) | 110 (31) |
| 40 - 59 | 75 (43) | 80 (46) | 155 (44) |
| 60 - 79 | 6 (3) | 71 (41) | 77 (22) |
| ≥ 80 | 0 (0) | 8 (5) | 8 (2) |
| Male gender, No. (%) | 123 (70) | 106 (61) | 229 (65) |
| Duration of chronic hepatitis B infection, months | 70 (13 - 510) | 84.5 (12 - 514) | 80.5 (12 - 514) |
| Quantitative HBV DNA, IU/mL | 17850 (< 10 - 295,000,000) | 601.5 (< 10 - 150,000,000) | 3470 (< 10 - 295,000,000) |
| Antiviral therapy at baseline, No. (%) | 32 (18) | 24 (14) | 56 (16) |
| Antiviral therapy during observation, No. (%) | 105 (60) | 100 (58) | 205 (59) |
| Laboratory parameters | |||
| Albumin [35 - 52 g/L] | 44 (23 - 69) | 42 (23 - 70) | 43 (23 - 70) |
| Aspartate aminotransferase (AST) [< 40 U/L] | 37 (6 - 1387) | 35.5 (9 - 704) | 36 (6 - 1387) |
| Alanine aminotransferase (ALT) [< 50 U/L] | 63 (8 - 1834) | 37.5 (5 - 783) | 47 (5 - 1834) |
| Gamma glutamyltransferase (GGT) [< 60 U/L] | 29 (3 - 612) | 32 (6 - 1714) | 31 (3 - 1714) |
| Total bilirubin [< 1.1 mg/dL] | 0.6 (0.2 - 18.7) | 0.7 (0.2 - 9.6) | 0.7 (0.2 - 18.7) |
| Platelet counts [150 - 400 × 109/L] | 231 (117 - 478) | 178 (29 - 339) | 202 (29 - 478) |
| Prothrombin time [70 - 120 %] | 98 (10 - 130) | 94 (34 - 135) | 97 (10 - 135) |
| Alpha fetoprotein (AFP) [< 5.7 kU/L] | 2 (0.8 - 197) | 3 (1 - 2030) | 3 (0.8 - 2030) |
| Liver cirrhosis, No. (%) | 6 (3) | 35 (20) | 41 (12) |
| Child A | 6 (3) | 26 (15) | 32 (9) |
| Child B | 0 (0) | 6 (3) | 6 (2) |
| Child C | 0 (0) | 3 (2) | 3 (1) |
| MELD score | 6 (6 - 9) | 8 (6 - 22) | 8 (6 - 22) |
| Fatty liver on imaging, No. (%) | 46 (26) | 69 (40) | 115 (33) |
| BMI, No. (%) | |||
| 0 - 24.9 | 110 (63) | 89 (51) | 199 (57) |
| 25 - 29.9 | 40 (22.7) | 66 (38) | 106 (30) |
| 30 - 50 | 26 (15) | 19 (11) | 45 (13) |
| Type 2 diabetes, No. (%) | 6 (3) | 20 (12) | 26 (7) |
| Arterial hypertension, No. (%) | 7 (4) | 51 (29) | 58 (17) |
| Alcohol consumption in g/d, No. (%) | |||
| 0 | 161 (92) | 166 (95) | 327 (93) |
| 1 - 39 | 9 (5) | 4 (2) | 13 (4) |
| ≥ 40 | 6 (3) | 4 (2) | 10 (3) |
| Follow-up, y | 9.5 (1 - 19.8) | 8.3 (1 - 21.3) | 8.9 (1 - 21.3) |
| Number of presentations | 8 (2 - 44) | 9.5 (2 - 62) | 8 (2 - 62) |
| HCC development during observation, No. (%) | 2 (1) | 21 (12) | 23 (6.6) |
Baseline Characteristics According to FIB-4 Groupsa
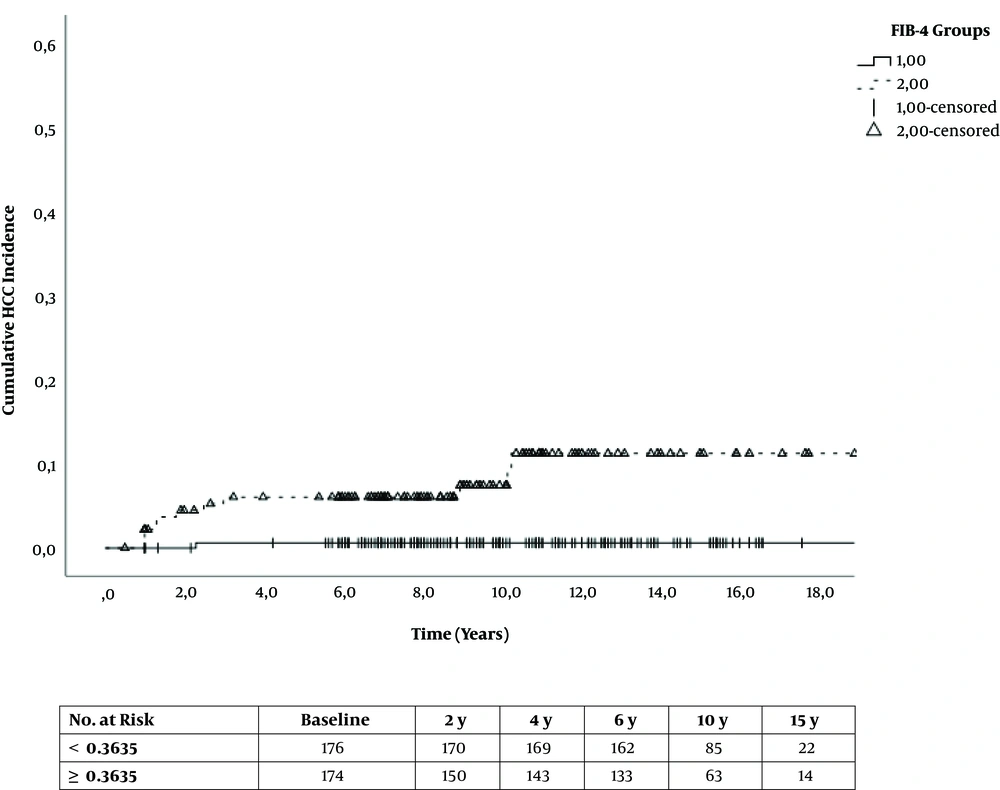
Compared to the FIB-4reference group, patients in the FIB-4elevated bracket showed a hazard ratio (HR) of 11.67 (95% CI: 2.73 - 49.96) for HCC incidence (P = 0.001). We adjusted for age, sex, type 2 diabetes, BMI, antiviral medication, amount of alcohol consumption, and duration of CHB infection, which led to an adjusted hazard ratio (aHR) of 7.90 (95% CI: 1.58 - 39.39) for HCC incidence in FIB-4elevated patients compared to the reference group (P = 0.012) (Table 3 and Figure 1).
| FIB-4 | Event (N) | HR (95% Confidence Interval) | P Value | aHRb (95% Confidence Interval) | P Value |
|---|---|---|---|---|---|
| All subjects (N = 350) | |||||
| < 0.3635 (N = 176) | 2 | 1 | 1 | ||
| ≥ 0.3635 (N = 174) | 21 | 11.67 (2.73 - 49.96) | P < 0.001 | 7.90 (1.58 - 39.39) | P = 0.012 |
Cox’s Proportional Hazards Models for HCC Incidencea
The sub-analysis of non-cirrhotic patients at baseline included 309 patients in total. In this cohort, 12 patients developed HCC, including one patient in the FIB-4reference (< 0.3635) group and the rest in the FIB-4elevated bracket (≥ 0.3635) group (Appendix 1 in Supplementary File).
Compared to the non-cirrhotic FIB-4reference group, patients in the non-cirrhotic FIB-4elevated bracket showed an HR of 15.88 (95% CI: 2.04 - 123.2) for HCC incidence (P < 0.0001). The adjusted HR (see above) was 11.99 (95% CI: 1.36 - 105.72) (P = 0.001) (Appendix 2 in Supplementary File). The Kaplan-Meier analysis for cumulative HCC incidence can be found in Appendix 3 in Supplementary File.
5. Discussion
This study aimed to evaluate whether the FIB-4 score, using modified cutoffs, can be applied in stratifying the risk of HCC development in non-Asian patients with CHB. After adjusting for age, sex, type 2 diabetes, BMI, antiviral medication, amount of alcohol consumption, and duration of CHB infection, FIB-4elevated patients showed an adjusted HR of 7.90 (95% CI: 1.58 - 39.39) for HCC incidence compared to the reference group (P = 0.012). Of the 23 HCCs diagnosed during the follow-up, 91% were developed in patients in the ‘elevated’ bracket.
Suh et al. previously investigated the possibility of using FIB-4 as a means of stratifying the HCC risk in patients with CHB. They carried out a retrospective cohort study, including 986 Korean HBsAg carriers (22). Patients with FIB-4 scores of 1.7 - 2.39 and ≥ 2.4 showed an adjusted HR of 4.57 (95% CI: 1.50 - 13.92) and 21.34 (95% CI: 7.73 - 58.92), respectively, regarding the incidence of HCC when compared to those with FIB-4 < 1.25. However, in a previous publication, we found that the cutoffs proposed by Suh et al. did not apply to our clinical setting.
There were several differences in baseline characteristics when comparing patients who developed HCC during observation to those who did not (Table 1). One interesting finding was a tendency towards lower median HBV DNA at baseline (10 IU/mL, range < 10 - 9,880,000 IU/mL; P = 0.068) in patients who developed HCC. It had previously been discussed in another publication on the management of CHB whether the suppression of HBV DNA led to an HCC risk reduction (29). Recent data suggest that a reduction in HBV DNA results in reductions in liver-related events and HCC development (30-32). On the other hand, in a multicenter study by Arends et al., 14 out of 744 CHB patients (in a Western population) were diagnosed with HCC. Twelve of these patients had achieved a virological response before (HBV DNA < 80 IU/mL) (33). Papatheodoridis et al. reported similar reports. They found that on-therapy virological remission did not significantly affect the HCC incidence in 818 Greek HBeAg-negative CHB patients (34). Taking these findings into account, it appears comprehensible that patients diagnosed with HCC during observation had lower HBV DNA levels. One further explanation is that at baseline, a portion of our patients who developed HCC had inactive hepatitis B, coupled with a trend towards more HCC patients under antiviral therapy at baseline (30% vs. 15%; P = 0.072)
At baseline, 16% of CHB patients were on antiviral treatment compared to only 4% published by Suh et al. (22). This increased rate most likely resulted in lower aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels in our collective leading to lower FIB-4 values overall. This suggests that the cutoffs published by Suh et al. (22) were set too high for patients with CHB receiving antiviral therapy. Parameters ALT and AST usually drop or even normalize under therapy, leading to a decrease in FIB-4 values consequently. To explore this further, we determined new FIB-4 cutoff values.
Current guidelines for the management of HCC recommend offering surveillance to patients with CHB, NASH (regardless of the presence of cirrhosis), or diagnosed cirrhosis aiming at detecting HCC at early, curable stages (7-11). It was demonstrated in a randomized controlled trial of almost 19,000 high-risk individuals that biannual screening can reduce HCC-related mortality by 37% (35).
It remains challenging to identify CHB patients who have a heightened risk for the development of HCC in spite of the known risk factors such as cirrhosis (12, 13), HCV coinfection (14, 15), HDV, or alcohol abuse (16). An effective and routinely applicable predictive tool is needed to improve the identification of CHB patients at high risk.
Even when removing the established primary risk factor of HCC development in CHB patients, i.e., cirrhosis, our proposed FIB-4 cutoffs continue to allow a statistically significant difference between high- and low-risk patients (supplementary material).
Therefore, a possible clinical application of FIB-4 (with modified cutoffs) as a tool for HCC risk stratification in non-Asian CHB patients could be its use as a tool of seeding out low-risk patients (FIB-4 < 0.3635) with the consequence of adjusting screening intervals. The current German and US HCC guidelines suggest an interval of six months for routine sonography in CHB-infected patients regardless of age and other risk factors (26, 36). Assuming that the individual FIB-4 value is determined regularly within recommended surveillance intervals, it could be argued that a more lenient screening strategy for ultrasound examinations with or without AFP measurements may be sufficient in a low-risk population, while in high-risk patients, a tight surveillance regimen with sonography and control of laboratory parameters is indispensable. According to our observations, the benefit of a tightly knit screening regimen is likely higher in the FIB4elevated cohort. The median FIB-4 value of patients with HCC was more than twofold higher (0.81 (0.10 - 3.76)) than the low-risk cutoff (< 0.3635). Furthermore, 91% of all observed HCCs had an elevated FIB-4 (≥ 0.3635). Only one percent of patients classified as low-risk due to their FIB-4 values developed HCC during observation, whereas 12% of patients classified as having an elevated risk developed HCC in the clinical course.
This study has some limitations. Because our analysis was strictly retrospective, the time intervals of follow-up visits could no longer be influenced. All patients who had only one or two short-time visits were excluded to establish a sufficient follow-up period. This led us to exclude 218 patients which may have resulted in a higher disease burden in our collective overall, explaining in part that the HCC incidence of 6.6% in our study population was relatively high. The annual HCC incidence is 4% - 6% in Asian CHB patients and approximately 1% in Caucasian CHB populations (37). The majority of HCCs in our analysis were diagnosed during the first three to four years of the observational period. Because these individuals were infected with CHB significantly longer at the point of study entry, this may relate to a lead-time bias. To determine the optimal cutoffs for the prediction of future HCC development, we only considered the individual FIB-4 values at baseline. However, when implementing FIB-4 as a risk stratification tool for the guidance of surveillance, regular measurements are mandatory to monitor changes in individual risk.
Instead of sourcing data from medical service claims, we extracted our pieces of patient data from medical records and they were analyzed by experienced physicians. This allowed for high-quality clinical data. Our datasets were complete for all factors of interest. They included the availability of HBV-DNA, HDV-RNA, HCV-RNA, and HIV-RNA. We decided to exclude confounding factors such as viral co-infection, HCC diagnosis at baseline, and patients with only short-time visits with the aim of acquiring a reliable and adjusted database for analysis. Even though this meant the exclusion of 43% of our initially identified CHB cohort, in our view, the quality of data was improved. Our follow-up was performed over a long period (median 8.9 years; range 1 - 21.3 years) with a loss to follow-up rate of only 7%.
We are the first to validate new cutoff values for the use of the FIB-4 index in the field of HCC risk stratification in a non-Asian CHB population. Its predictive value lies in the discrimination of low and elevated HCC risk; the ≥ 0.3635 cutoff placed 91% of HCCs in the FIB4elevated group while low values could serve as a clinical indicator for a low likelihood of HCC incidence in non-Asian patients with CHB. This analysis underlines the fact that regional differences in risk and therapy need to be taken into account when implementing screening tools and that it can be useful to question and test the validity of standard values in different patient populations.
5.1. Conclusions
The discussion regarding screening recommendations for HCC in CHB remains controversial, partially due to concerns about the quality and lack of existing evidence (7, 8, 38). Considering that HCC surveillance is costly and its benefit is frequently discussed, effective and routinely applicable predictive tools are needed, which may help guide surveillance, especially in regions where CHB and HCC prevalence is high while health care resources are limited. An ideal tool would be noninvasive, easy to apply and analyze, inexpensive, reproducible, and reliable in predicting the risk of HCC development. Furthermore, it should be able to monitor changes in the individual risk in real-time so that a clinician can intervene timely and change the established surveillance strategy. According to our data, FIB-4, when applying specific cutoffs, can be such a candidate tool. Further studies in geographically and ethnically different populations need to be carried out to clarify the role that FIB-4 may play in the prediction of HCC incidence in CHB patients worldwide.