1. Background
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease, from simple hepatic steatosis to steatohepatitis, which may progress to cirrhosis and eventually to hepatocellular carcinoma. NAFLD is a problem of high morbidity and mortality related to the liver, and also an increase in mortality due to cardiovascular disease (CVD) and cancer (1). Serum alanine aminotransferase (ALT) is a sensitive biomarker of hepatic injury commonly used to screen and detect abnormal liver function and estimate levels of abnormality. The increase in serum ALT level is more closely related to fat accumulation in the liver and reveals histological progression of the liver. Higher serum ALT level accompanied by echogenic liver ultrasonography, in the absence of any identified cause of liver disease, suggests the diagnosis of NAFLD (2, 3).
Despite well-known risk factors for NAFLD such as genetic components and life style, the underlying mechanism of fatty liver is unclear (2, 4, 5). However, environmental influences such as car engine exhaust particles, metals, and various polychlorinated elements are significant causes of NAFLD progression (6-8). The effect of exposure to air pollutants on the onset of diseases is widely accepted, and reveals a difference in the composition of fatty acids in the liver and adipose tissue that consequently has negative effects on health by increasing the risk of cardiovascular disease, systemic and immune inflammation, and symptoms of depression (9-11). The harmful impact of air pollutants is involved in the pathogenesis of fatty liver from oxidative stress and insulin resistance leading to increased levels of aminotransferase (12).
Delta-aminolevulinic acid dehydratase (ALAD) is a cytosolic sulfhydryl enzyme strongly inhibited by lead airborne particulates and generally attributed to the pathogenesis of lead poisoning (13, 14). Human ALAD is a polymorphic enzyme, encoded by the ALAD gene on chromosome 9q34 and involved in the synthesis of heme by converting aminolevulinate (ALA) to porphobilinogen (PBG). The common variant 177G>C (rs1800435) in the exon 4 of ALAD, which substitutes asparagine with lysine on residue 59, produces two codominant alleles ALAD1 and ALAD2 in three genotypes of ALAD 1-1, ALAD 1-2, and ALAD 2-2 (13, 15). It is shown that carriers of the ALAD2 (C allele) are prone to exhaust particles to have higher blood lead concentrations than the frequent ALAD1 (G allele). The electronegative properties of the ALAD2 enzyme increase its affinity for lead (16, 17).
2. Objectives
Atmospheric pollution as a major concern in urban environments affects patients with NAFLD; therefore, the current study aimed at investigating the frequency of ALAD genotypes, the enzyme related to air pollution that increases the sensitivity to lead poisoning, in patients with NAFLD compared to healthy individuals and the association of the ALAD rs1800435 polymorphism with serum ALT level.
3. Methods
3.1. Study Subjects
A total of 300 subjects (179 males and 121 females) in a prospective cohort at a referral clinic affiliated to Tehran University of Medical Sciences were enrolled in the current study. The current nested case-control study was conducted on 100 patients with NAFLD and 200 subjects with normal ALT levels (< 40 U/L in males, < 34 U/L in females) as a control group selected consecutively. The fatty liver index (FLI) algorithm was used to diagnose NAFLD according to the formula published by Huang et al. (18). Selection of ALT threshold values was based on previous studies to estimate the upper health limits in healthy blood donors (3). The study included subjects without a history of alcohol abuse, autoimmune hepatitis, use of hepatotoxic drugs, evidence of viral liver disease, tumors, cholestasis, or other metabolic diseases of the liver. Venous blood samples after a 12-hour overnight fasting were collected from all participants. Demographic data were obtained and the biochemical parameters for each subject were tested using available standardized methods (19). The study protocol was in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Digestive Disease Research Institute (DDRI), Shariati Hospital, Tehran University of Medical Sciences (TUMS) (ethical code: 416/780). Written informed consent was obtained from all subjects.
3.2. Genotyping for the ALAD Polymorphism
Genomic DNAs were extracted from the blood samples using the Gentra Puregene kit (Qiagen, Alameda, CA, USA) according to the manufacturer’s recommendations. To identify the two variants of ALAD, the polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) with specific primers to detect MspI restriction sites (C|CG G) was performed as described previously (15). The primers used in the ALAD genotyping were: 5’-AGACAGACATTAGCTCAGTA-3’ and 5’-GGCAAAGACCACGTCCATTC-3’ in amplification of a 916-base-pair (bp) sequence. PCR products were digested with the MspI restriction enzyme to produce dissimilar fragments that lead to specific genotypes. The fragmented products were then analyzed on the agarose gel. The wild type ALAD1-1, homozygous variants ALAD 2-2 and ALAD 1-2 heterozygous were defined by fragments of 582, 511, and 582 bp in addition to 511 bp, accordingly. The protocol and the condition of the PCR were as previously described (15).
3.3. Laboratory Measurements
Serum insulin was measured by ELISA (the enzyme-linked immunosorbent assay) technique (Diesse Company, Italy). Lipid profiles, including total cholesterol (TC), triglycerides (TG), high-density lipoprotein-cholesterol (HDL-c), liver enzymes, and fasting plasma glucose were tested using an autoanalyzer (Cobas c 702, Roche; Shanghai, China). Platelets were counted using Sysmex kx-21.
3.4. Statistical Analysis
Analysis of variance (ANOVA) was used to compare continuous variables. Chi-square test was used to compare categorical group variables and also determine if the genotype distribution was in the Hardy-Weinberg equilibrium. Logistic regression analysis was used to calculate the odds ratios of the variants for ALT level. SPSS version 15.0 (SPSS, Chicago, IL, USA) was used to analyze data and P < 0.05 was considered significant.
4. Results
4.1. Clinical Features of the Study Population
The general characteristics of the NAFLD and non-NAFLD groups are shown in Table 1. The number of females and males, mean age, and body mass index (BMI) in the NAFLD group were respectively 42 and 58 subjects, 41.1 ± 14.2 years, and 24.7 ± 5.4 kg/m2 compared to 79 and 121 subjects, 11.44 ± 2.99 years, and 23.98 ± 4.6 kg/m2, which were not significantly different. The mean ALT, ALP, TG, cholesterol, and serum insulin levels were significantly higher in the NAFLD group than in the non-NAFLD group (P < 0.001).
| Characteristics | Patients with NAFLD (N = 100) | Controls (N = 200) | P Value |
|---|---|---|---|
| Age, y | 42.3 (11.9) | 41.1 (14.2) | 0.48 |
| Gender, female/male | 42/58 | 79/121 | 0.70 |
| BMI, kg/m2 | 24.7 (5.4) | 23.98 (4.6) | 0.18 |
| Platelet × 109/L | 304.42 (89.01) | 293.70 (77.42) | 0.25 |
| ALT, IU/L | 42.7 (8.3) | 16.9 (5.7) | 0.000 |
| ALP, IU/L | 192.5 (55.6) | 169.6 (66.8) | 0.03 |
| Cholesterol, mg/dL | 188.4 (40.7) | 165.0 (33.6) | 0.000 |
| HDL, mg/dL | 45.4 (10.1) | 47.6 (10.0) | 0.075 |
| TG, mg/dL | 198.3 (153.5) | 112.0 (52.7) | 0.000 |
| FBS, mg/dL | 98.4 (18.6) | 85.6 (19.4) | 0.092 |
| Insulin, IU/mL | 9.50 (5.56) | 7.86 (6.24) | 0.027 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; FBS, fasting blood sugar; Hb, hemoglobin; HDL, high-density lipoprotein; TG, triglyceride.
aValues are expressed as mean (SD).
4.2. ALAD Genotypes and Allele Distribution
According to the findings, 10% of the patients with NAFLD were ALAD2 carriers with both ALAD 2-2 (1%) and ALAD 1-2 (9%) genotypes. This rate was 6.5% in the control group, all with ALAD 1-2 genotype, without significant differences (P > 0.09).
The frequency of the C-allele of ALAD rs1800435 was 5.5% in patients and 3.3% in controls, with a borderline difference (P = 0.07); however, both the G- and the C-alleles were in the Hardy-Weinberg equilibrium (P > 0.05). Table 2 revealed the allelic frequency of the ALAD rs1800435 polymorphism and the genotype distribution between the patients and controls.
| Variantsa | Patients with NAFLD (N = 100) | Controls (N = 200) | P Value | ||
|---|---|---|---|---|---|
| ALAD 177G>C (rs1800435) | 0.07 | ||||
| Genotype | N | GF (%) | N | GF (%) | |
| GG | 90 | 90 | 187 | 93.5 | |
| CG | 9 | 9 | 13 | 6.5 | |
| CC | 1 | 1 | 0 | 0 | |
| Allele | N | AF (%) | N | AF (%) | |
| G (ancestral) | 189 | 94.5 | 387 | 96.7 | |
| C (minor) | 11 | 5.5 | 13 | 3.3 | |
aGenotypic and allelic frequencies are shown as absolute and percentage data.
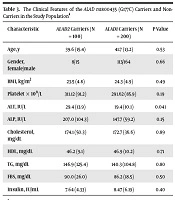
To assess whether 177G>C (rs1800435) polymorphism influences clinical parameters; the mean ± standard deviation (SD) of the variables was compared between the carriers and non-carriers of ALAD rs1800435. As shown in Table 3, the serum ALT level was considerably higher in the ALAD2 carriers than in non-carriers of ALAD2 (29.4 ± 13.9 vs. 19.4 ± 10.1, P = 0.041). However, no significant differences were observed in other experimental features and demographic data among the study groups (P > 0.05). Using the linear regression adjusted for age, BMI, and gender, a significant association was observed between the ALAD2 genotype and the ALT level. For ALAD rs1800435, each C-allele increased the ALT level by 1.24 IU/L (95% confidence interval (CI): 0.22 - 2.67; P = 0.04).
| Characteristic | ALAD2 Carriers (N = 100) | ALAD1 Carriers (N = 200) | P Value |
|---|---|---|---|
| Age, y | 39.6 (15.4) | 41.7 (13.2) | 0.53 |
| Gender, female/male | 8/15 | 113/164 | 0.66 |
| BMI, kg/m2 | 23.5 (4.8) | 24.3 (4.9) | 0.49 |
| Platelet × 109/L | 311.12 (91.2) | 291.62 (85.9) | 0.19 |
| ALT, IU/L | 29.4 (13.9) | 19.4 (10.1) | 0.041 |
| ALP, IU/L | 207.0 (104.3) | 147.7 (59.2) | 0.15 |
| Cholesterol, mg/dL | 174.1 (50.3) | 172.7 (36.6) | 0.89 |
| HDL, mg/dL | 46.2 (9.1) | 46.9 (10.2) | 0.71 |
| TG, mg/dL | 146.9 (125.4) | 140.3 (104.8) | 0.80 |
| FBS, mg/dL | 90.0 (26.0) | 86.2 (18.5) | 0.50 |
| Insulin, IU/mL | 7.64 (4.33) | 8.47 (6.19) | 0.40 |
aValues are expressed as mean (SD).
5. Discussion
The effects of environmental factors such as air pollution on the incidence of NAFLD along with an increase in liver enzyme levels and consequent steatosis were previously reported (6, 9, 20). Exposure to diesel exhaust particles in diabetic obese mice is positively associated with NAFLD, and mortality due to diabetes mellitus is probably through increased oxidative stress (10). This situation is important to explore the contribution of variants in ALAD gene related to lead toxicity in common diseases such as NAFLD. Although the ALAD rs1800435 polymorphism has important effects on the susceptibility to toxicity of lead particle, information on the distribution of ALAD gene polymorphism in NAFLD subjects is not provided. Furthermore, there was no evidence to demonstrate the distribution of genetic variants of the ALAD genotypes in the Iranian population.
The current study results showed that the distribution of ALAD genotypes in patients with NAFLD compared to healthy subjects had no significant differences and also allelic variations of ALAD locus showed similar frequencies in both study groups. Previous studies confirmed that ALAD2 carriers are generally more likely to have a high blood lead level (14, 21, 22); however, blood lead was not measured in the current study. Though, carriers of ALAD 177G>C variants in the current study showed an increase in serum ALT; therefore, it could be evidence for an association between ALAD genotypes and predisposition to NAFLD. The serum ALT level is a sensitive indicator and one of the key tests to recognize, screen, and follow-up the patients with hepatitis. The significance of ALT activity as an index of liver damage was examined in previous studies (23, 24). Furthermore, the allelic frequency of ALAD in the current study was very similar to that of previously reported in Caucasian and Asian populations with distribution of 92% for ALAD 1-1 and 8% for ALAD 1-2 (22, 25). Therefore, the current study results suggested a consistency in the distribution of ALAD 177G>C (rs1800435) variants in the Iranian population.
5.1. Conclusions
In conclusion, although there was no difference in the distribution of ALAD genotypes among the patient groups with controls; however, ALAD2 carriers had a higher serum ALT level. Air pollution has the most important effects on human health, causing numerous diseases and leading to high morbidity and mortality, especially in the developing countries. Therefore, with reference to the hypothesis that air pollution influences the development of NAFLD, as an important economic burden and health problem, it must be assessed by measuring the level of lead in blood and through mechanisms related to systemic oxidative stress.