1. Background
In much of sub-Saharan Africa, the prevalence of hepatitis b virus (HBV) infection is high, with most HBV infections occurring in early childhood (1). Botswana has been described by the World Health Organization (WHO) to have an intermediate prevalence of HBV (2). Between 5% and 10% of human immunodeficiency virus (HIV)-infected patients in Botswana have a positive hepatitis B surface antigen (HBsAg) result (3, 4) and the prevalence of HBsAg is 2.1% among pregnant women (HIV positive or negative) (5). Occult HBV infection is defined as having negative HBsAg but positive HBV DNA. We previously reported a 6.6% prevalence of occult HBV in pregnant women in Botswana (5).
HBsAg positivity has been associated with adverse pregnancy outcomes in a small number of studies. For example, low birth weight was previously associated with maternal HBsAg positivity (6). HBsAg positivity has also been associated with high rates of preterm delivery, premature rupture of the membranes, placental abruption, stillbirth, and cesarean delivery (7, 8). To our knowledge, no data exist on the association between occult HBV and pregnancy outcomes.
2. Objectives
Therefore, we assessed the potential associations between having either a positive HBsAg or occult HBV infection at delivery, and pregnancy and infant health outcomes.
3. Methods
3.1. Study Population
We used the existing data and stored samples from the Tshipidi study, an observational study of the effects of in-utero exposure to maternal HIV or to antiretroviral drugs on neurodevelopmental and child health outcomes at two years of age between 2010 and 2012. We enrolled 454 HIV-infected and 458 HIV-uninfected pregnant women and their live-born infants (9). The research was done following the Declaration of Helsinki guidelines, and the research protocol was approved by the Health Research Development Committee at the Botswana Ministry of Health and Wellness (HRDC00524) and the Office of Human Research Administration at the Harvard T.H. Chan School of Public Health (18093).
3.2. HBV Testing
Maternal HBV-positive samples previously screened were used (5). One stored plasma sample from each participant taken at the delivery time was evaluated for HBsAg using Murex HBsAg kit (Murex Biotech, Dartford, UK) and HBV DNA using COBAS® AmpliPrep/COBAS® Taqman® HBV Test V.2.0 (Roche Diagnostics, Mannheim, Germany) with a lower limit of detection of 20 IU/mL. The testing was done at the Botswana Harvard HIV Reference Laboratory (BHHRL) following the methods described previously (5).
3.3. Statistical Analysis
The exposure of interest was the occurrence of either HBsAg+ positivity or occult HBV infection, defined as having a negative HBsAg but detectable HBV DNA. When either of these tests was positive, the mother was considered to have “HBsAg+/occult HBV”. The pregnancy outcomes of interest were preterm birth at less than 37 weeks of estimated gestational age and stillbirth. The infant health outcomes of interest were low birthweight or hospitalization by 24 months after birth. Binary logistic regression was used to explore the possible impact of maternal HBV status on each outcome, adjusted for maternal HIV status, ART used during pregnancy, and maternal age. STATA V14.0 was used for the analysis and P values of < 0.05 were considered statistically significant.
4. Results
Out of 912 women enrolled in the Tshipidi study, 752 samples from 384 HIV-positive and 368 HIV-negative women were available for additional HBV testing. Of the 752 women, 16 (2.1%) were HBsAg-positive, and 41 of 622 (6.6%) had occult HBV. The proportions of women with any of these positive HBV tests did not differ by HIV status. Of the 384 HIV-positive women, 253 were on zidovudine (AZT), 112 were on highly active antiretroviral therapy (HAART), 4 took peripartum prophylaxis, one did not take any antiretroviral treatment (ART), and 9 had missing ART data. Among the 57 mothers who were HBsAg+ or had occult HBV infection, 29 were HIV-positive. Of the 29 HIV-positive women, 10 received HAART, 18 received AZT, and one received maternal peripartum prophylaxis. Of the 12 HIV positive women with overt HBV, only were four on HBV HAART and 8 were on AZT monotherapy. Of the 17 HIV-positive, occult HBV-infected women, 6 received a HAART containing regimen and 11 were on AZT monotherapy for the prevention of mother to child therapy (PMTCT). The HAART regimens the women were on contained either lamivudine, tenofovir and/or emtricitabine with anti-HBV properties.
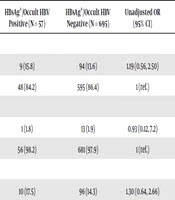
As shown in Table 1, the rates of preterm birth, stillbirth, infant hospitalization, and low birth weight were similar in women with and without HBsAg positivity/occult HBV. When evaluating the association between maternal HBsAg positivity/occult HBV and infant health outcomes, no statistically significant differences were observed between the HBsAg+/occult HBV positive and negative pregnant women. Low birth weight was associated with higher odds of outcome (> 1), whilst infant hospitalization was associated with lower odds of outcome (< 1); however, this was not statistically significant between HBV-positive and HBV-negative women. Adjustment for maternal age and HIV status did not substantially alter these findings. Analysis as per the type of infection, occult versus overt infection, was also done (see Appendices 1 and 2 in Supplementary File) and no significant associations were found between HBV infection and stillbirth, preterm birth, low birth weight, or infant hospitalization.
| Outcome | HBsAg+/Occult HBV Positive (N = 57) | HBsAg+/Occult HBV Negative (N = 695) | Unadjusted OR (95% CI) | P Value | Adjusted ORc (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Preterm birth, wk | 0.65 | 1.06 (0.48, 2.37) | 0.88 | |||
| < 37 | 9 (15.8) | 94 (13.6) | 1.19 (0.56, 2.50) | |||
| ≥ 37 | 48 (84.2) | 595 (86.4) | 1 (ref.) | |||
| Stillbirth | 0.95 | 1 | ||||
| Yes | 1 (1.8) | 13 (1.9) | 0.93 (0.12, 7.2) | |||
| No | 56 (98.2) | 681 (97.9) | 1 (ref.) | |||
| Low birthweight, kg | 0.47 | 1 | ||||
| < 2.5 | 10 (17.5) | 96 (14.3) | 1.30 (0.64, 2.66) | |||
| ≥ 2.5 | 47 (82.5) | 588 (84.7) | 1 (ref.) | |||
| Infant hospitalizationd | 0.19 | 1 | ||||
| Yes | 6 (11.8) | 120 (17.4) | 0.56 (0.23, 1.32) | |||
| No | 51 (89.5) | 569 (82.6) | 1 (ref.) |
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; OR, odds ratio.
aValues are expressed as No. (%).
bThe n for the variables differs slightly; preterm birth (n = 689), stillbirth (n = 694), low birth weight (n = 684), and infant hospitalization (n = 689).
cAdjusted for maternal age, HIV status, and ART regimen during pregnancy.
dInfant hospitalization by 24 months of age.
5. Discussion
There were no differences in the rates of preterm birth, stillbirth, low birth weight, or child hospitalization by maternal HBsAg positivity/occult HBV status. Prior studies have shown various results with regard to the association between active maternal HBV and preterm birth. A number of studies have found similar rates of preterm birth in HBV-positive women and HBV-negative women (7, 10). On the other hand, several studies have reported that positive maternal HBsAg status is associated with preterm birth (8, 11, 12). A possible explanation is that HBV DNA is found in the placenta and trophoblast cells in HBV-infected women and thus it is hypothesized that this may exaggerate the placental inflammatory response, which is a contributor to preterm birth (13). As reported elsewhere, we did not observe an association between maternal HBV status and stillbirth (7). Cui et al. (7) reported that miscarriage (but not stillbirth) was significantly associated with maternal HBV status when compared to HBV-negative mothers. Miscarriage and stillbirth are usually caused by other negative outcomes during pregnancy, which include pregnancy-induced hypertension, fetal distress, and macrosomia (14). We could not determine the rates of miscarriage in this dataset as the data were not collected in the parent study.
Maternal HBV status was not associated with low birth weight in this study, which is in line with several other studies (7, 10, 15). However, Safir et al. (16) reported that maternal hepatitis infection was a risk factor for low birth weight; but, it should be noted that hepatitis B and C (HCV) infections were pooled together in that study, making it impossible to determine whether the positive association was due to HBV or HCV. Maternal HBsAg and HBeAg positivity were found to be associated with increased odds of low birth weight of infants (6). Finally, we found no evidence of the impact of maternal HBsAg positivity/occult HBV infection on infant hospitalization and we are not aware of prior published literature evaluating this question.
Most HAART regimens in Botswana contain either tenofovir (TDF), lamivudine (3TC), or emtricitabine (FTC). These antiretrovirals have anti-HBV properties and could have resulted in the lower HBV prevalence in our cohort (5). A study by Anderson et al. showed that there were 38% HBsAg losses 24 months of post-Truvada-based cART initiation (17). There is, therefore, a good possibility that the HBV prevalence rates in this study were low, as 29.2% of the HIV-infected women screened for HBV were on HAART that is HBV suppressive.
In summary, in our study, maternal occult/HBsAg+ HBV infections were not found to cause any adverse pregnancy or infant health outcomes. Our study had several limitations, most importantly the relatively small sample size along with several relatively rare outcomes. Other studies previously assessed other factors such as gestational diabetes, pre-eclampsia, and placental abruption, which we were unable to evaluate (10, 14).