1. Background
Hepatitis B virus (HBV) is an important infectious agent, responsible for chronic hepatitis B (CHB) infection. CHB infection can lead to liver diseases, such as cirrhosis and liver cancer, and therefore, it is considered a global health concern (1). Several host, viral, and exogenous factors can affect the natural course of CHB infection. Host factors include the age of disease onset, gender, and immunological status, viral factors comprise viral genotypes, viral mutations, and viral replication rates, and finally, exogenous factors incorporate alcohol consumption and intake of special foods producing hepatic toxins (2, 3). Generally, the detection of hepatitis B surface antigen (HBsAg) is a reliable method for the diagnosis of HBV infection (4).
Evidence shows that in the natural course of HBV, spontaneous HBsAg clearance may occur (seroclearance), while in more than 50% of these patients, hepatitis B surface antibody (anti-HBs) may be produced (seroconversion). In the event of seroclearance or seroconversion, HBsAg may be cleared or reduced to levels below the detection limit. Previous findings show that in a small percentage of HBV patients, HBV DNA may be detected in the liver, plasma, or peripheral blood mononuclear cells (PBMCs). In these cases, DNA is detected using ultra-sensitive molecular detection techniques, thus occult HBV infection (OBI) is diagnosed (4-6). Also, anew definition of OBI suggests the presence of HBV DNA without detect able HBsAg, with or without anti-HBs antibodies. In OBI, HBV DNA is present in the liver tissues while PBMCs (< 200 IU/mL) in the presence or absence of HBV DNA in the serum (7).
Several mechanisms may lead to the progression of disease to OBI, including the integration of viral DNA into the host chromosome, HBV S gene mutation, window period after acute HBV infection, immunodeficiency, and immune complexes hiding HBsAg (8, 9). OBI may relapse in some cases and reactivate the virus. Accordingly, the importance of OBI has been emphasized in recent years (10). Many studies indicate that OBI is an important risk factor for hepatocellular carcinoma (11, 12). In fact, OBI may preserve most of the oncogenic characteristics of an overt HBV infection, as it can be integrated into the host genome, synthesizes transforming proteins even at low concentrations, and causes a mild but persistent necroinflammation (13).
The prevalence of OBI in HBsAg-serocleared and -seroconverted patients has not been clearly reported in the medical literature (14). In chronic hepatitis B patients that has been HBsAg serocleared, with or without anti HBs, the DNA of virus may still be detectable.
2. Objectives
Therefore, this study was conducted to determine the prevalence of HBV DNA in the serum and PBMCs of new chronic HBV infected individuals as HBsAg-serocleared (HBsAg-, Anti-HBs-) and old chronic HBV infected individuals as HBsAg-seroconverted (HBsAg-, Anti-HBs+) group.
3. Methods
3.1. Study Sample
In this follow-up study, a total of 1116 patients with CHB, who were referred to our private clinic, were followed-up for 10 years on average. HBsAg and anti-HBs were measured twice over the past six months. Sixty patients were selected based on their previous laboratory tests and divided into two groups: HBsAg-serocleared and HBsAg-seroconverted. Both groups were negative for HBsAg. Patients with antibody concentrations ≥ 10 mIU/mL were considered as seroconverted, while cases with antibody concentrations ≤ 10 mIU/mL were considered as serocleared. Patients with HIV or HCV co-infection and immunocompromised patients were excluded from the study; none of these patients were treated using anti-viral drugs.
All patients agreed to participate in this study. The study protocol was approved by the Ethics Committee of Babol University of Medical Sciences (number: MUBABOL.REC.1395.157).
3.2. Sample Collection and Processing
About 5 mL of peripheral blood was drawn from each patient into sterile ethylenediaminetetraacetic acid (EDTA)-containing vacationer tubes, and then, plasma was separated and frozen at -20ºC until use. PBMCs were isolated by Ficoll density gradient centrifugation using Lymphodex (Inno‐Train, Germany). After washing with phosphate-buffered saline (pH, 7.3 ± 0.1) in triplicate, the cells were stored at -20ºC.
3.3. Serological Tests
Serological markers of HBV (HBsAg, anti-HBs, HBeAg, and anti-HBc) were measured by ELISA assay (Dia. Pro Diagnostic Bioprobes, Milan, Italy), based on the manufacturer’s instructions.
3.4. DNA Extraction
HBV DNA was extracted from a 200-µL plasma aliquot and 3 – 5 × 106 of PBMCs, using Favor prep Blood Genomic DNA Extraction Mini Kit (Favorgen Biotech Corp., Taiwan), according to the manufacturer’s instructions. Murine cytomegalovirus (MCMV; fast-track diagnostics, Luxembourg) was used as internal control. Moreover, sterile microcentrifuge tubes, containing only the reaction mixtures, were processed simultaneously with plasma and PBMC samples as the negative control.
3.5. Quantitative Real-Time Polymerase Chain Reaction (Q-PCR) of HBV DNA
HBV DNA in the plasma and PBMC samples were measured by Q-PCR assay (fast-track diagnostics, Luxembourg), according to the manufacturer’s protocols. Amplification was also carried out using a Rotor-Gene® QRT-PCR system (Qiagen GmbH, Hilden, Germany).
3.6. Statistical Analysis
Data were analyzed in SPSS version 22. Kaplan-Meier was used to estimate and plot the cumulative survival probabilities. The effects of important factors were evaluated using Cox proportional hazards model. Furthermore, crude and adjusted hazard ratios (HRs) were reported at 95% confidence interval. A P values less than 0.05 was set as a significant association of difference based on the type of statistical test.
4. Results
4.1. Sample Selection
Thirty HBsAg-negative patients, who were anti-HBs- and anti-HBc-positive (seroconverted group), as well as 30 HBsAg-negative patients, who were anti-HBc-positive with an anti-HBs level < 10 mIU/mL (serocleared group), were evaluated in this study. The demographic characteristics of the patients are presented in Table 1.
| Serocleared Group | Seroconverted Group | |
|---|---|---|
| Mean age ± SD, y | 50.5 ± 13.1 | 49 ± 11 |
| Sex | ||
| Male | 22 | 16 |
| Female | 8 | 14 |
| Route of transmission | ||
| Needlestick | 1 | 0 |
| Contaminated razor | 0 | 1 |
| Tattoo | 0 | 1 |
| Blood transfusion | 1 | 2 |
| Mother to child | 7 | 7 |
| Dental services | 2 | 5 |
| Unknown | 19 | 14 |
Serology viral markers were retested using ELISA. In the first group (HBsAg-serocleared), three patients became HBsAg-positive, while in the second group (HBsAg-seroconverted), HBsAg was positive and anti-HBs was negative in two patients.
4.2. Real Time PCR
DNA of virus was found in the PBMC and plasma in one out of 5 patients that became HBsAg positive. However, in 4 others, DNA was found only in the plasma. Besides HBV DNA was detected in the PBMCs of 4 (14.8%) out of 27 serocleared patients (HBsAg- and Anti-HBs+) and 3 (10.7%) out of 28 seroconverted patients (HBsAg- and Anti-HBs+) (occult HBV patients). None of these 55 patients were positive for HBV DNA in the plasma.
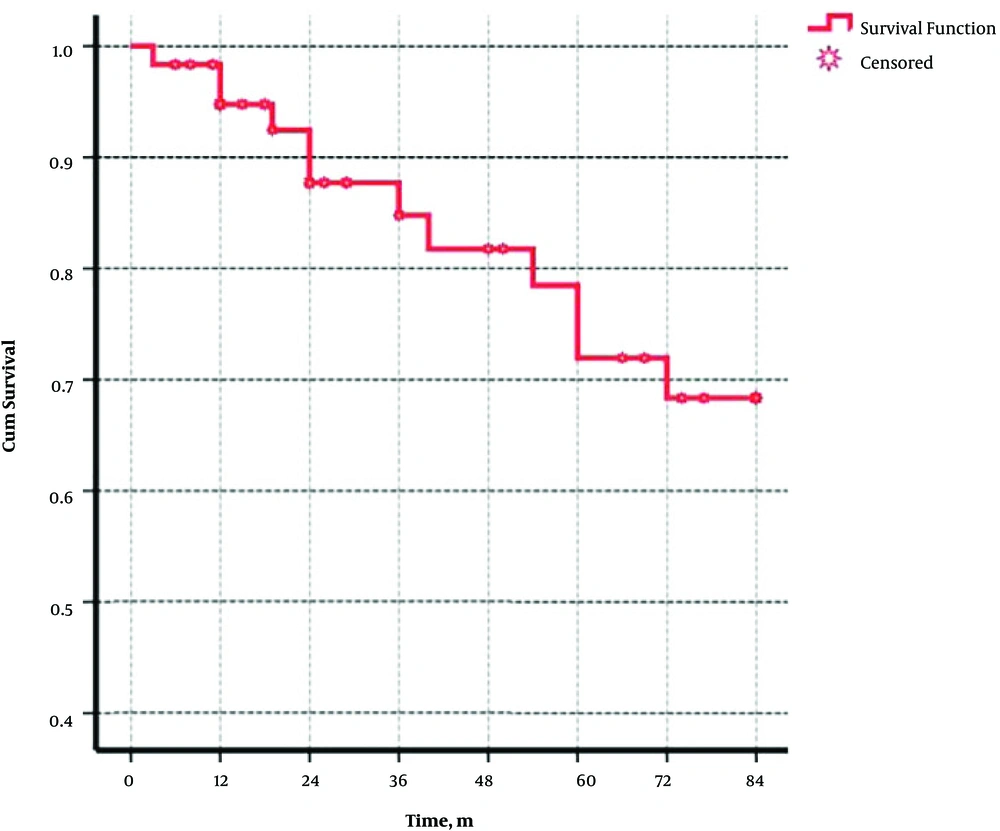
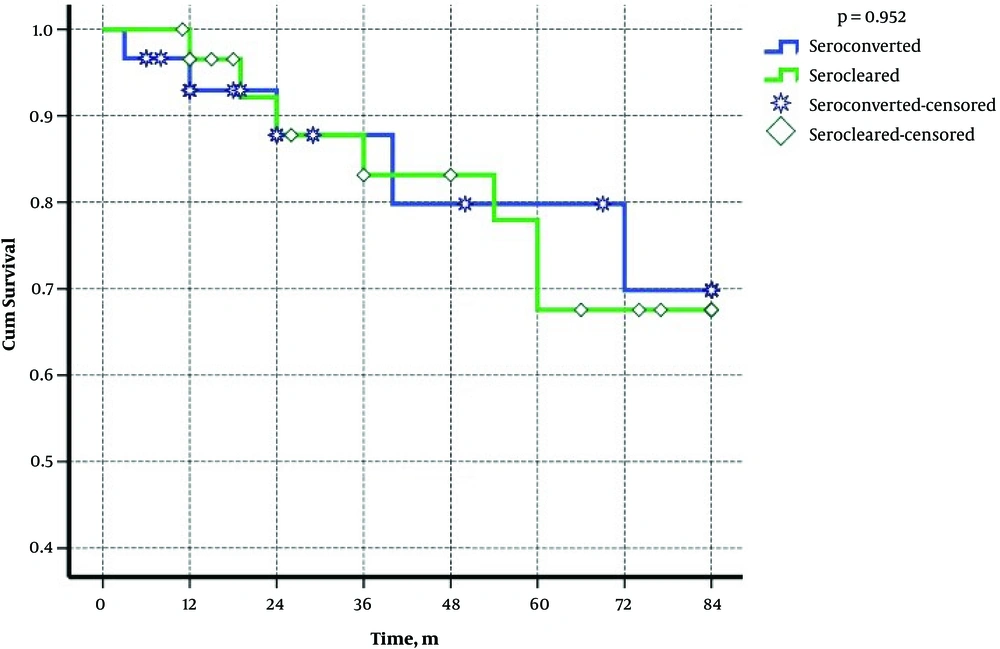
Table 2 demonstrates the demographical, serological, and virological characteristics of patients in two groups. Cox regression showed that age, gender, duration of disease and serological situation of patients had no effects on relapse of the disease (Table 3). Plus, the probabilities of reactivation in these two groups were 6%, 13%, and 16% after 12, 24, and 36 months of seroclearancy, respectively (Figure 1). Figure 2 shows that the cumulative rate of HBV reactivation in serocleared and seroconverted groups is not different.
| Study Group (Case Number) | Age | Gender | HBsAg | HBV DNA in PBMC, IU/mL | HBV DNA in Plasma, IU/mL | Anti-HBc | Anti-HBs Titer, IU/mL |
|---|---|---|---|---|---|---|---|
| Serocleared | |||||||
| 5 | 41 | F | - | 534.6 | 0 | + | 1.7 |
| 7 | 46 | M | - | 83.7 | 0 | + | 3.2 |
| 8 | 77 | M | - | 121.5 | 0 | + | 1.7 |
| 21 | 53 | F | + | 0 | 17.550 | + | 6.9 |
| 24 | 56 | M | - | 555 | 0 | + | 7.3 |
| 25 | 55 | F | + | 0 | 1395 | + | 0.1 |
| 29 | 55 | M | + | 0 | 395.55 | + | 1.3 |
| Seroconverted | |||||||
| 16 | 37 | F | + | 0 | 2174 | + | 0.6 |
| 18 | 62 | F | - | 143 | 0 | + | 107.5 |
| 25 | 39 | M | - | 17 | 0 | + | 47.5 |
| 28 | 56 | M | + | 2621.7 | 28644 | + | 8.8 |
| 30 | 61 | F | - | 348 | 0 | + | 230.6 |
Abbreviations: Anti-HBc, antibody against hepatitis B core antigen; Anti-HBs, antibody against hepatitis B surface antigen; F, female; HBsAg, hepatitis B surface antigen; M, male; PBMC, peripheral blood mononuclear cells.
| Variables | Crude HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|
| Serocleared | 1.036 (0.327 - 3.281) | 0.953 | 1.082 (0.316 - 3.706) | 0.900 |
| Femal) | 1.672 (0.527 - 5.304) | 0.383 | 2.498 (0.641 - 9.729) | 0.187 |
| Age, y | 1.015 (0.967 - 1.065) | 0.551 | 1.015 (0.966 - 1.067) | 0.553 |
| Duration of disease, y | 1.030 (0.966 - 1.098) | 0.366 | 1.048 (0.975 - 1.127) | 0.200 |
5. Discussion
Occult HBV infection is a potential risk factor for cirrhosis and liver cancer. However, there is inconsistent information regarding its prevalence in different parts of the world. Diagnosis and prevalence of this disease can be effective in preventing its transmission (15). In this study, 60 individuals with negative HBsAg that were found during the 10-years follow up were assessed. 5 (8.3%) patients were diagnosed as positive HBsAg over time, suggesting the possibility of disease recurrence, despite negative HBsAg, and in some cases, even after antibody formation. Among the 55 patients with negative HBsAg, HBV DNA was detected in the PBMCs of 7 (12.7%) patients.
Since patients with negative serological tests had significant viral DNA in their PBMCs or serum, serological tests were not sufficient to determine if the patient could transmit the virus; therefore, it is suggested to use molecular methods instead of serological tests. False OBI is reported if the level of DNA exceeds 200 IU/mL in patients with negative HBsAg; also, S gene mutation can lead to the negation of HBsAg, despite the high viral DNA load. Some researchers have categorized OBI into two types of seropositive (anti-HBc alone or together with anti-HBs) and seronegative (no antibodies); like seroconverted and serocleared groups that were defined in our study (7). Both groups included some patients with HBV DNA level above 200 IU/mL, with a possible mutation. By detecting the mutant gene, in addition to serological tests, we can identify such cases and effectively prevent disease transmission by the carrier.
In a study conducted in Malaysia (15), the prevalence of OBI was reported to be 5.5% among blood donors. Considering the significant number of OBI patients, a more accurate screening should be carried out in blood transfusions. This study also showed that all patients with positive anti-HBc were also positive for HBV DNA; they concluded that anti-HBc could be used for screening of OBI. However, the results of the present study showed that patients with positive anti-HBc are not necessarily positive for viral DNA, which is consistent with the results reported by Shahmoradi et al. in Iran (16).
In another study, in spite of negative serological tests in some patients, viral DNA was detected in the plasma, which was attributed to viral DNA mutation (17). Similarly, in our study, DNA was found in peripheral lymphocytes, even in patients with high levels of antibodies. Recent studies have shown that evaluation of HBeAg- and HBsAg-negative patients in long-term follow-ups, precise assessment of viral load, and measurement of the initial HBsAg level can be effective in preventing relapse (18). In our study, viral load in some HBsAg-negative patients was above 200 IU/mL in the long-term follow-up; therefore, time to relapse can be shorter in these patients, compared with others. Evidence suggests that the low level of HBsAg and viral load can be important, even for the patient’s therapeutic plan (19).
It is unknown if high anti-HBs level in OBI is associated with the increased risk of virus transmission. Theoretically, a high anti-HBs titer should be sufficient for neutralizing viral infectivity, whereas, in reality, viral DNA can be transmitted even if the antibody titer is high (20). Although anti-HBs level ≥ 10 IU/mL represents immunity, anti-HBs level ≤ 100 IU/mL cannot neutralize the infectivity of gene products containing viral DNA; this is especially important in immunocompromised donors when they have high anti-HBs titers (21). In our study, despite the high level of antibodies in some patients, viral DNA was detected in PBMCs, which could be due to S gene mutation.
Similarly, to what frequently happens in immunocompromised HBsAg-positive subjects, but less commonly, reactivation in chronic hepatitis B virus infection (HBV) may occur in OBI patients and may subsequently develop fulminant hepatitis. It is called spontaneous relapse during the course of the disease (22).In Karajibani et al.’s study 4.5% relapse was shown and in males was 2.53 times higher than females. In this study, age has no effect on relapse (23) that is similar to our study’ results. In our study, age, sex had no effects on the seroclearance or seroconversion of patients. In some cases, age has been used as a predictor of reactivation after treatment which may be due to the power of immune system at different ages (24).
There are several limitations in the present study such as the lack of quantitative measurement of antigen S, lack of information about HBV DNA in liver tissue and non-assessment of hepatic fibrosis.
5.1. Conclusions
Taken together, in patients with CHB, who became HBsAg negative in long-term follow-ups, there is a possibility of disease recurrence whether the serum antibody is formed or not. Recurrence may be predicted considering the viral load in PBMCs. Moreover, given the possibility of DNA transfer, blood donation should be done with caution in these individuals.