1. Background
Hepatitis B virus (HBV) makes a significant health problem (1). Chronic HBV infection may evolve to cirrhosis and liver decompensation. Hepatocellular carcinoma is the most drastic complication (1, 2).
Liver fibrosis (≥ F2) is the main parameter to decide on the start of treatment (1). Liver fibrosis currently can be diagnosed invasively using a liver biopsy or noninvasively using laboratory and radiological tools. Liver biopsy is the gold standard and can detect other diseases such as steatosis but is threatening. Transient elastography measurement by Fibroscan is an easy noninvasive approach that can be done at bedside but is costly and not available in all centers (3). The laboratory approaches can be used for the direct measurement of fibrosis markers or formulae models. The formulae can be based on simple routine tests such as Fibrosis-4 (FIB-4) test or complex non-routine labs such as FibroTest (3).
2. Objectives
This study aimed to assess the usefulness of fibrosis index, FIB-4, King’s fibrosis score, albumin-bilirubin (ALBI) score, gamma-glutamyl transferase-platelets (GPR), and gamma-glutamyl transferase-albumin (GAR) ratio compared to transient elastography as diagnostic models of the extent of liver fibrosis, both pre- and post-treatment.
3. Methods
This study enrolled 217 patients diagnosed with HBV-related chronic liver disease, attending the HBV Clinic at the National Liver Institute Hospital, Menoufia University, Egypt. The informed consent was obtained from all enrolled patients after obtaining the institutional review board approval (IRB 00002314, May 2009). The study lasted from January 2010 to December 2017.
All patients were diagnosed to have chronic hepatitis B infection based on persistent positivity for HBsAg for more than six months (1). They were either positive (n = 21) or negative (n = 196) for HBeAg. The fibrosis grades (F1 to F4) were determined using Transient Elastography (TE) measurement by FibroScan in the supine position after 6 - 8 hours of fasting (4, 5). The F4 grade was defined as liver stiffness of > 14.5 KPa (6).
The exclusion criteria were decompensated cirrhosis, HCV infection, autoimmune hepatitis, primary biliary cholangitis, alcohol consumption, and HIV disease. Full history-taking and physical examination were done. Baseline labs including liver function test, renal function test, CBC, albumin, INR, and serum HBV DNA level were obtained on treatment, at various intervals, and at the end of the follow-up. The choice of treatment regimen depended on the financial and different local insurance protocols, which included pegylated interferon (n = 1), lamivudine (n = 139), lamivudine-adefovir combination (n = 4), entecavir (n = 15), and tenofovir (n = 58).
The non-invasive models were calculated for the quantification of fibrosis at pre- and post-treatment (end of the follow-up period of the study), as follows:
FIB-4 score = [age (years) × AST (U/L)]/[number of platelets (109/L) × ALT (U/L)(1/2)] (7)
King’s score = age × AST (U/L) × INR/platelet count (109/L) (8)
Fibrosis index score = 8 - 0.01 × number of platelets (109/L) - albumin (g/dL) (9)
ALBI score = -0.085 × albumin (g/L) + 0.66 × log (bilirubin μmol/L) (10)
GPR = [GGT (U/L)/ULN of GGT]/[platelet count (109/L) × 100] (11)
GAR = GGT (IU/L)/albumin (g/L) (12)
3.1. Statistical Analysis
Data were statistically analyzed using IBM® SPSS® version 21 for Windows (IBM Corporation, North Castle Drive, Armonk, New York, USA) and MedCalc® version 18.2.1 (Seoul, Republic of Korea). Data are expressed as mean ± standard deviation for normally distributed data, median (interquartile range) for data that lacked normal distribution, and number (percentage) for nominal data. Comparisons between the two groups were made using the Student’s t-test for parametric data and the Mann-Whitney test for nonparametric data. Comparisons of the variable changes in the same group were made using Wilcoxon test for nonparametric data. Comparisons between multiple groups were made by the ANOVA test for normally distributed data. The chi-squared test (χ2) and Fisher exact test were used for categorical data analysis. Univariate and multivariate binary logistic regression was done for detecting the independent predictors of fibrosis. The receiver operating characteristic (ROC) curve analysis was used for the detection of the cutoff value of the fibrosis assessment models. The ROCs of different fibrosis assessment models were compared using the DeLong test to assess the variable discrimination.
4. Results
The study prospectively enrolled 217 patients diagnosed with HBV chronic liver disease. The average age was 37.47 ± 10.15 years. The participants were mainly males (73.7%), treatment naïve (77.4%), and with non-F4 fibrosis (79.7%).
Forty-nine (22.6%) patients were treatment-experienced, including 14.3% pegylated interferon, 59.2% lamivudine, 18.4% lamivudine-adefovir combination, and 8.2% entecavir. The average duration of the follow-up was 33.20 ± 20.94 years.
Patients positive for HBeAg were younger and had higher HBV DNA levels than the HBeAg negative group (Table 1). Both groups had comparable values for transient elastography, fibrosis index score, FIB-4 score, King’s fibrosis score, ALBI score, GPR, and GAR.
| HBeAg | Total (N = 217) | P Value | ||
|---|---|---|---|---|
| Negative, N = 196 (90.3%) | Positive, N = 21 (9.7%) | |||
| Age | 37 (4.2) | 29 (7.44) | 36 (17) | 0.001 |
| Male/female | 142/54 | 18/3 | 160/57 | 0.189 |
| Naïve/experienced | 164/32 | 4/17 | 168/49 | 0.001 |
| Non-F4/F4 fibrosis | 155/41 | 18/3 | 173/44 | 0.472 |
| HBV DNA × 105 IU/mL | 9 (42) | 5000 (20072) | 12 (88) | 0.001 |
| Transient elastography (kPa) | 6 (3.25) | 7 (5) | 6.9 (5) | 0.260 |
| Treatment duration | 36 (36) | 36 (39.5) | 36 (36) | 0.970 |
| Fibrosis index score | -36.16 (6.02) | -34.97 (5.03) | -36.16 (5.5) | 0.416 |
| FIB-4 score | 1.01 (1.07) | 0.85 (0.44) | 1.01 (0.95) | 0.436 |
| King’s fibrosis score | 6.85 (7.43) | 7.53 (3.47) | 6.95 (7.34) | 0.618 |
| ALBI score | -2.9 (0.12) | -2.8 (0.3) | -2.87 (0.5) | 0.268 |
| GPR | 0.43 (0.27) | 0.4 (0.26) | 0.42 (0.27) | 0.751 |
| GAR | 1.13 (0.44) | 1.08 (0.51) | 1.13 (0.45) | 0.818 |
Patients with advanced fibrosis (F4) were older than non-F4 fibrosis patients, and were chiefly males, as shown in Table 2. They also had higher values (P = 0.001) of fibrosis index score, FIB-4 score, King’s fibrosis score, ALBI score, GPR, and GAR unlike HBV DNA values. Patients with ALT levels of < 2 folds and ≥ 2 folds had a comparable (P > 0.05) fibrosis index score, FIB-4 score, ALBI score, GPR, and GAR. In contrast, King’s fibrosis score was lower in patients with ALT levels of below 2 folds (6.62 vs. 15.2, P = 0.001). Patients aged ≥ 40 years had significantly higher values (P = 0.001) of fibrosis index score (-35.50 vs. -36.72), FIB-4 score (1.79 vs. 0.81), King’s fibrosis score (11.39 vs. 4.91), ALBI score (-2.77 vs. -2.91), GPR (0.51 vs. 0.40), and GAR (1.22 vs. 1.08) than a younger age group.
| Liver Fibrosis | P Value | ||
|---|---|---|---|
| Non-F4, N = 173 (79.7%) | F4, N = 44 (20.3%) | ||
| Age | 35 (14.25) | 45 (15) | 0.001 |
| Male/female | 135/38 | 25/19 | 0.004 |
| Naïve/experienced | 133/40 | 35/9 | 0.706 |
| HBV DNA × 105 IU/mL | 17 (114.5) | 2 (13) | 0.001 |
| Treatment duration | 36 (25.5) | 14 (26.5) | 0.001 |
| Fibrosis index score | -37.12 (4.96) | -30.95 (7.29) | 0.001 |
| FIB-4 score | 0.88 (0.62) | 2.64 (2.32) | 0.001 |
| King’s fibrosis score | 5.66 (4.49) | 17.99 (20.94) | 0.001 |
| ALBI score | -3 (0.5) | -2.4 (0.7) | 0.001 |
| GPR | 0.39 (0.19) | 0.91 (0.68) | 0.001 |
| GAR | 1.07 (0.35) | 1.51 (0.58) | 0.001 |
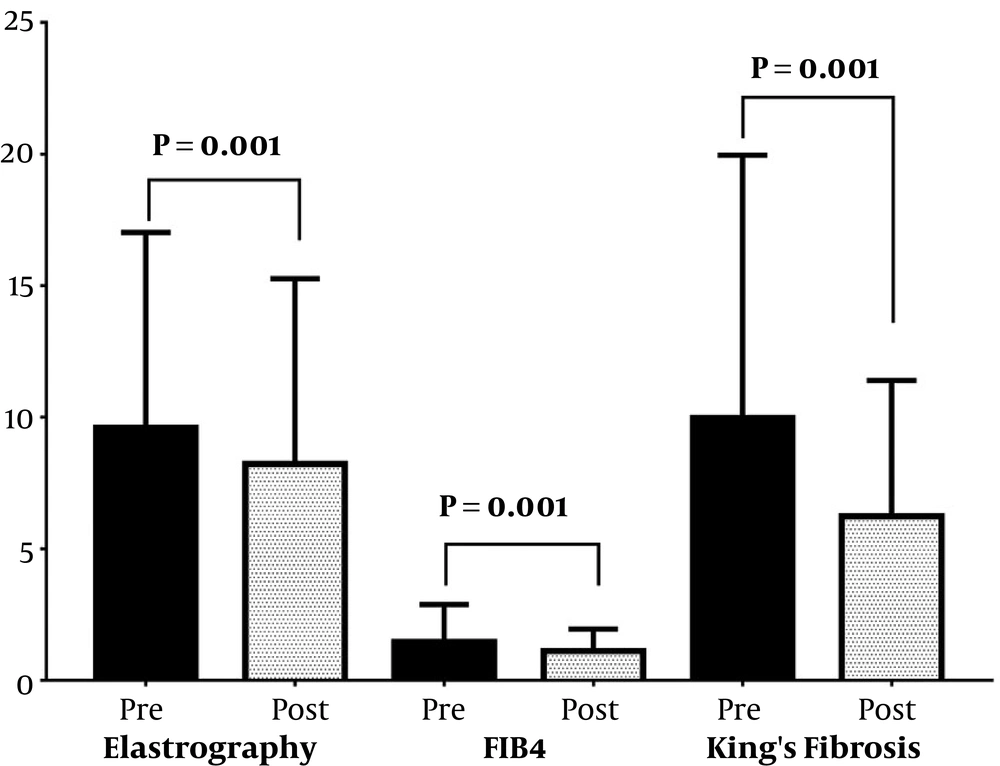
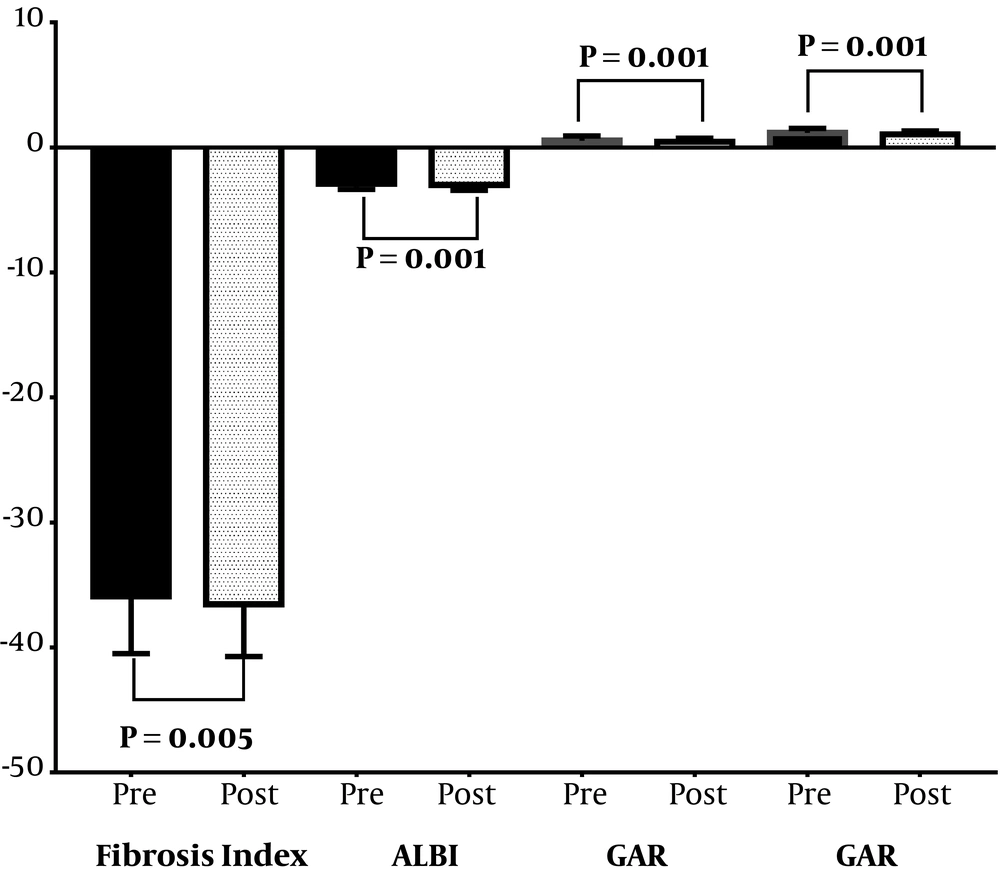
The HBV treatment was beneficial and decreased the fibrosis grades (P < 0.05) where the fibrosis assessment parameter values decreased from baseline to the end of the follow-up period (Figures 1 and 2), as follows: transient elastography from 6.90 to 5.90 with a median change of -1.00 kPa, fibrosis index score from -36.16 to -36.78 with a median change of -0.78, FIB-4 score from 1.00 to 0.79 with a median change of -0.17, King’s fibrosis score from 6.95 to 4.24 with a median change of -1.93, ALBI score from -2.87 to -3.02 with a median change of -0.10, GPR from 0.43 to 0.39 with a median change of -0.06, and GAR from 1.13 to 1.05 with a median change of -0.13. Patients who achieved a virological response (negative serum HBV DNA) at the end of the follow-up period were comparable (P > 0.05) to those who neither achieved nor maintained response respecting the mean changes in transient elastography, fibrosis index score, FIB-4 score, King’s fibrosis score, ALBI score, GPR, and GAR.
The ROC curve results demonstrating the best cutoff values for detecting ≥ F2 and F4 fibrosis are summarized in Table 3 for each of the fibrosis assessment models. On the comparison of the area under the ROC (AUROC) curve of different fibrosis assessment models for ≥ F2 fibrosis detection, it was found that fibrosis index score, FIB-4 score, King’s fibrosis score, ALBI score, and GPR were comparable (P > 0.05). The GAR was inferior to GPR (P = 0.035) but comparable to the rest of the scores (P > 0.05). On comparison of the AUROC curve of the different fibrosis assessment models for F4 fibrosis detection, it was found that fibrosis index score, FIB-4 score, King’s fibrosis score, ALBI score, and GPR were comparable (P > 0.05). The GAR was inferior to King’s fibrosis score (0.011), FIB-4 score (P = 0.019), and GPR (P = 0.02) but comparable to the rest of the scores (P > 0.05).
| Fibrosis Index | FIB.4 | King's Fibrosis | ALBI | GPR | GAR | |
|---|---|---|---|---|---|---|
| ≥ F2 Fibrosis | ||||||
| Cutoff | > -34.67 | > 1.13 | > 7.91 | > -2.7 | > 0.42 | > 1.21 |
| AUC | 0.694 | 0.739 | 0.725 | 0.698 | 0.727 | 0.664 |
| 95% CI | 0.628 - 0.754 | 0.676 - 0.797 | 0.661 - 0.783 | 0.632 - 0.758 | 0.663 - 0.785 | 0.597 - 0.727 |
| P | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Sensitivity, % | 47.2 | 78.9 | 59.26 | 42.6 | 69.44 | 51. |
| Specificity, % | 83.5 | 78.9 | 78.9 | 91. | 66.97 | 74.3 |
| PPV, % | 73.9 | 74.2 | 73.6 | 83.6 | 67.6 | 66.7 |
| NPV, % | 61.5 | 67.2 | 66.2 | 61.7 | 68.9 | 60.9 |
| F4 fibrosis | ||||||
| Cutoff | > -32.66 | > 1.88 | > 7.93 | > -2.7 | > 0.69 | > 1.28 |
| AUC | 0.838 | 0.895 | 0.903 | 0.843 | 0.870 | 0.803 |
| 95% CI | 0.782 - 0.884 | 0.846 - 0.932 | 0.856 - 0.939 | 0.788 - 0.889 | 0.818 - 0.912 | 0.744 - 0.854 |
| P | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Sensitivity, % | 63.64 | 72.73 | 90.91 | 70.45 | 70.5 | 72.73 |
| Specificity, % | 91.33 | 91.33 | 73.99 | 86.13 | 94.2 | 78.61 |
| PPV, % | 65.1 | 68.1 | 47.1 | 56.4 | 75.6 | 46.4 |
| NPV, % | 90 | 92.9 | 97 | 92 | 92.6 | 91.9 |
Abbreviations: AUC, area under the curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Fibrosis index score, FIB-4 score, ALBI score, GPR, and GAR had comparable F2 AUROC between patients positive and negative for HBeAg, unlike King’s fibrosis score (0.978 vs. 0.718, P = 0.001). Fibrosis index score, FIB-4 score, King’s fibrosis score, GPR, and GAR had comparable F4 AUROC between patients positive and negative for HBeAg, unlike ALBI score (0.963 vs. 0.838, P = 0.020).
Table 4 shows the independent predictors of F2 and F4 fibrosis. The independent predictors of F2 fibrosis by univariate analysis (Table 4) were the age, being HBeAg negative, fibrosis index score, FIB-4 score, King’s fibrosis score, ALBI score, and GPR. The GPR was associated with the highest odds ratio (odds = 48.3). On multivariate analysis, being HBeAg negative and FIB-4 score were the independent predictors of F2 fibrosis. The independent predictors of F4 fibrosis by univariate analysis were the age, female sex, fibrosis index score, FIB-4 score, King’s fibrosis score, ALBI score, and GPR. In fact, both the ALBI score and GPR had the highest odds ratios. On multivariate analysis, only was GPR the independent predictor.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| P | Odds | 95% CI | P | Odds | 95% CI | |
| F2 Fibrosis | ||||||
| Age | 0.001 | 1.05 | 1.02 - 1.08 | 0.112 | 0.96 | 0.92 - 1.01 |
| Female | 0.615 | 1.17 | 0.64 - 2.14 | |||
| Naïve | 0.273 | 1.43 | 0.75 - 2.72 | |||
| HBV DNA | 0.402 | 1.00 | 1.00 - 1.00 | |||
| HBeAg negative | 0.048 | 2.71 | 1.01 - 7.28 | 0.033 | 3.85 | 1.12 - 13.23 |
| Fibrosis index | 0.001 | 1.20 | 1.12 - 1.29 | 0.714 | 0.97 | 0.80 - 1.16 |
| FIB-4 score | 0.001 | 3.94 | 2.38 - 6.51 | 0.022 | 5.31 | 1.28 - 22.06 |
| King’s fibrosis | 0.001 | 1.16 | 1.09 - 1.23 | 0.411 | 0.96 | 0.86 - 1.07 |
| ALBI score | 0.001 | 5.56 | 2.71 - 11.43 | 0.219 | 2.94 | 0.53 - 16.37 |
| GPR | 0.001 | 48.30 | 10.22 - 228.24 | 0.244 | 3.63 | 0.41 - 31.83 |
| F4 Fibrosis | ||||||
| Age | 0.001 | 1.09 | 1.05 - 1.12 | 0.603 | 1.02 | 0.95 - 1.08 |
| Female | 0.005 | 2.7 | 1.35 - 5.42 | 0.332 | 1.7 | 0.58 - 5.01 |
| Naïve | 0.706 | 1.17 | 0.52 - 2.64 | |||
| HBV DNA | 0.862 | 1 | 1 - 1 | |||
| HBeAg negative | 0.476 | 1.59 | 0.45 - 5.65 | |||
| Fibrosis index | 0.001 | 1.41 | 1.27 - 1.57 | 0.766 | 0.94 | 0.64 - 1.38 |
| FIB.4 score | 0.001 | 5.01 | 3.06 - 8.21 | 0.723 | 0.77 | 0.19 - 3.19 |
| King’s fibrosis | 0.001 | 1.23 | 1.15 - 1.32 | 0.105 | 1.12 | 0.98 - 1.28 |
| ALBI score | 0.001 | 39.06 | 11.84 - 128.87 | 0.247 | 9.37 | 0.21 - 415.5 |
| GPR | 0.001 | 228.81 | 39.36 - 1330.13 | 0.027 | 17.25 | 1.39 - 214.22 |
5. Discussion
Hepatitis B virus is a worldwide health problem. In high prevalence areas, HBV-related liver disease is a common indication for liver transplantation. Hepatitis B virus may affect other organs. It is a common cause of glomerulonephritis and end-stage renal disease. Occult HBV may flare with immunosuppressive medications such as rituximab; thus, HBV should be tested before chemotherapy and biological therapy (1, 13). There is no curative therapy for HBV and the aim of treatment is to suppress the virus. The decision on treatment depends on the transaminases level, HBV DNA level, and the degree of liver fibrosis (≥ F2 or not) (1).
Earlier findings (14-16) were in favor of APRI and FIB-4 whereas recent studies (17, 18) found that both had moderate sensitivity. We search for new fibrosis models because FibroScan is not available in all hospitals; it is expensive, and needs regular maintenance. As a result, we still need routine investigations based on simple, cheap, accurate, and reliable models. The WHO recommends two to three cheap laboratory investigations in resource-limited settings (19).
In an earlier study by Lemoine et al. (11), GPR was superior to APRI and FIB-4. Some studies were in agreement (20-23) and others were in disagreement (24-26). DP et al. (27) conducted the only study comparing patients positive and negative for HBeAg. In HBeAg-negative patients, GPR was better than FIB-4 but they were comparable in HBeAg-positive patients.
Both King’s score and Fibrosis index score have been tested mainly in hepatitis C patients. Albumin-bilirubin score was used to assess liver dysfunction in HCC patients (10). It is better than the MELD score for assessing the mortality in hepatitis B patients (28).
The current study was conducted on 217 patients including 90.3% HBeAg-negative and 77.4% naive. Our study tested the previously studied models in patients with hepatitis B. We also tested some models for the first time in HBV patients including fibrosis index score, King’s fibrosis score, and ALBI score. Most of the previous studies were conducted in the Asian population.
In our study, The transient elastography, fibrosis index score, FIB-4 score, ALBI score, GPR, and GAR were not affected by ALT elevation above 2 folds in contrast to King’s fibrosis score that was higher in patients with an ALT level above two folds. All the studied models had higher values in patients older than 40 years. Only Wang et al. (22) found higher values of GPR with age, AST, and bilirubin elevation.
We found only Lemoine et al.’s study (11) to be similar to our study. It was conducted mainly in patients who were negative for HBeAg, while other studies enrolled all positive (21) or both patients (20, 27, 29, 30). Another point is that most previous studies were conducted in China but our study and the study by Lemoine et al. (11) were conducted in Africa. There are differences in the prevalent HBV genotype, BMI, and environmental factors. The cutoff value ranged from 0.32 to 0.46 for F2 and from 0.56 to 0.93 for F4 in various studies (11, 20-23, 29, 30).
Concerning the best model for F2 discrimination, the fibrosis index score, FIB-4 score, King’s fibrosis score, ALBI score, and GPR were similar. The GAR was inferior to GPR but comparable to the rest of the scores (P > 0.05). Concerning F4 discrimination, the fibrosis index score, FIB-4 score, King’s fibrosis score, ALBI score, and GPR were the same. The GAR was inferior to King’s fibrosis score, FIB-4 score, and GPR but comparable to the rest of the scores.
We also tried to answer the question “Are the fibrosis models affected by the presence of HBeAg?” By comparison of the AUROC curve for F2 discrimination, the fibrosis index score, FIB-4 score, ALBI score, GPR, and GAR were similar while King’s fibrosis score had higher values in patients positive for HBeAg. Fibrosis index score, FIB-4 score, King’s fibrosis score, GPR, and GAR had comparable F4 AUROC between patients positive and negative for HBeAg, unlike ALBI score (0.963 vs. 0.838, P = 0.020). The only published study in this regard was done by DP et al. (27). On the comparison of patients positive and negative for HBeAg for F2 discrimination, GPR was comparable in both groupsbut FIB-4 had higher values with positivity. Regarding F4 discrimination, GPR and FIB-4 were the same. All the studied models were the independent predictors of F4 fibrosis but the ALBI score and GPR had the highest odds ratios.
To our knowledge, this is the first study that reported the follow-up data at post-treatment using liver fibrosis models. All the patients that underwent treatment had improved fibrosis model values by the end of the follow-up period. The new point here is that all patients whether with or without virological response experienced decreasing fibrosis model values, as well as the transient elastography values.
It is known that the treatment of HBV leads to the reversal of liver fibrosis, as shown in various studies (31-34). In an earlier study, resistance to lamivudine caused the progression of fibrosis [34]. Whether the reversal of fibrosis needs just the suppression of the virus (decreasing viral load) or negativity of the virus (negative serum HBV DNA) is under question. Schiff et al. (31) in a small study (n = 10) report that just the long-term suppression of the virus by entecavir could decrease fibrosis stages. At the endpoint of their study, all patients were positive for HBV DNA but with lower levels than pre-treatment values.
Marcellin et al. (32) in a large study followed patients on tenofovir treatment for up to five years. The progression of liver fibrosis (96%) and regression of cirrhosis (74%) stopped in patients with undetected HBV DNA (< 400 copy/mL). In other words, most patients had suppressed viruses as the lower limit of detection of the used apparatus was 169 copies/mL. Therefore, the question arises “Will the suppression of the virus by drugs lead to improved liver fibrosis?”
This was a single-center study. Other limitations included the small number of patients, non-randomization, lack of HBV genotyping, liver biopsy, and the choice of treatment based on different insurance reimbursement policies.
5.1. Conclusions
The GPR, fibrosis index, King’s fibrosis score, ALBI, and FIB-4 are useful diagnostic models of liver fibrosis in HBV patients.