1. Background
Drug-induced hepatotoxicity is a major concern among physicians prescribing medications. Among various drugs, acetaminophen is the most common drug associated with liver injury, followed by Amoxicillin/clavulanic acid (1). Pharmacologically, the likelihood of liver injury can be idiosyncratic or predictable by the cumulative dose of the drug. The induced injury could be accompanied by increased transaminases and cholestasis with elevated bilirubin, or a combination of both (2). Liver injury has been reported in up to 6% of patients receiving chemotherapy and suffering from underlying liver disease increases the risk of drug induced hepatotoxicity up to 18% of them that lead to a change in treatment regimen in 60% of cases (3). One of the liver injuries associated with chemotherapy or hormone therapy is non-alcoholic fatty liver or hepatosteatosis (4). Fatty liver refers to the accumulation of adipocytes in hepatocytes that becomes steatohepatitis if left untreated and accompanied by degeneration of hepatocytes. Chemotherapy drugs such as 5FU, platinum derivatives, and taxanes have shown to induce oxidative stress in normal hepatocytes leading to the accumulation of these lipid globules and hepatic steatosis (5, 6).
Gastrointestinal cancer patients are often treated with regimens containing 5FU, oxaliplatin, and irinotecan. On the other hand, patients with breast cancer are often treated with chemotherapy regimens containing doxorubicin, cyclophosphamide, and paclitaxel and receive tamoxifen if their hormone receptors are positive. Studies have shown that both of these treatment regimens are associated with the risk of liver injury (7-11).
2. Objectives
The present study was conducted to determine the prevalence of fatty liver in breast and gastrointestinal cancer patients during and after chemotherapy and to investigate its associated risk factors.
3. Methods
This cross-sectional study was performed on 152 gastrointestinal malignancy and breast cancer volunteer patients undergoing chemotherapy referring to the Oncology Clinic of Birjand University of Medical Sciences in 2016 - 2017, prospectively. The inclusion criteria included patients over 18 years of age, with gastrointestinal or breast cancers, and/or undergoing hormone therapy or chemotherapy. Patients with the age of fewer than 18 years, having liver metastasis and chronic liver disease or viral hepatitis were excluded. The project protocol was approved by the Ethics Committee of the Medical School of Birjand University of Medical Sciences (IR.BUMS.REC.1397.122). Written informed consent was obtained to enter the study. All patients underwent ultrasound for fatty liver examination before chemotherapy. Then, patients underwent four to six months of chemotherapy (based on clinical diagnosis and selected chemotherapy protocol). Ultrasound was again performed on patients after treatment.
All abdominal sonography were performed by a same faculty member using the Voluson General Electric apparatus. For span evaluation of the liver, upper and lower limits were determined in the midclavicular line and its size was calculated using a regular fixed ruler. The subcostal view of the right upper abdominal quadrant at a coronal cross-section in the axillary line was used to evaluate liver parenchyma. Fatty liver was determined based on the echogenicity of liver parenchyma at the level of fat echo and the visibility rate of the portal vein and hepatic vein. Then, in deep inspiration, the diameter of the portal vein was measured and the diagnosis was confirmed by ultrasound (Table 1) (12).
| Grade | Details |
|---|---|
| Grade I | The liver’s echogenicity increases slightly and the diaphragm and intrahepatic arteries are normal. |
| Grade II | The liver’s echogenicity is moderately elevated and the diaphragm and intrahepatic vessels fade slightly. |
| Grade III | The liver’s echogenicity increases intensely and the diaphragm and intrahepatic arteries and the posterior portion of the liver's right lobe fade or become obscured. |
Ultrasound Criteria for Fatty Liver Severity
The sample size was estimated at 140 patients based on the results by Chu et al. (13) considering d = 0.2, p = 0.45, q = 0.55, Z = 1.96, and α = 0.05 using the formula.
To increase the power of the study, 152 patients were enrolled.
Using SPSS 10 software (SPSS Inc., USA), descriptive statistics including mean, standard deviation, and frequency were reported. The chi-square and Fisher tests were used to determine the prevalence of fatty liver according to demographic data and the presence of metabolic syndrome and diabetes. The McNemar test was used to compare the frequency distribution of fatty liver occurrence during and after chemotherapy. A significance level of 5% was considered.
4. Results
A total of 152 patients were enrolled, including 85 patients with breast cancer and 67 patients with gastrointestinal cancer. Most patients were in the age group of 45 - 54 years (48 cases, 31.6%). The chemotherapy regimen of all breast cancer patients was doxorubicin and cyclophosphamide, followed by paclitaxel (AC-T). The majority of the patients with breast cancer were treated by tamoxifen after chemotherapy (56 cases, 65.9%). The chemotherapy regimen was FOLFOX in 64.2% of the patients with gastrointestinal cancer (containing 5FU, leucorin, and oxaliplatin, mainly used in colon cancer patients) and ECF in 35.8% of the patients (containing 5FU, leucorin, and epirubicin, mainly used in gastric cancer patients). Table 2 presents the patients’ demographic information.
| Total, No. (%) | BC, No. (%) | GIC, No. (%) | |
|---|---|---|---|
| Age | |||
| 15 - 24 | 1 (0.7) | 1 (1.2) | 0 |
| 25 - 34 | 11 (7.2) | 8 (9.4) | 3 (4.5) |
| 35 - 44 | 25 (16.4) | 17 (20) | 8 (11.9) |
| 45 - 54 | 48 (31.6) | 34 (40) | 14 (20.9) |
| 55 - 64 | 30 (19.7) | 14 (16.5) | 16(23.9) |
| 65 - 74 | 24 (15.8) | 8 (9.4) | 16 (23.9) |
| 75 - 84 | 12 (7.9) | 3 (3.5) | 9 (14.3) |
| 85 - 94 | 1 (0.7) | 0 | 1 (1.5) |
| Sex | |||
| Female | 103 (67.8) | 85 (100) | 18 (26.8) |
| Male | 49 (32.2) | 0 | 49 (73.2) |
| BMI | |||
| Underweight | 20 (13.2) | 6 (7.1) | 14 (20.9) |
| Normal weight | 86 (56.6) | 45 (52.9) | 41 (61.2) |
| Overweight | 33 (21.7) | 24 (28.2) | 9 (13.4) |
| Obese | 13 (8.6) | 10(11.8) | 3 (4.5) |
| Diabetes | |||
| Positive | 5 (3.3) | 1 (1.2) | 4 (6) |
| Metabolic syndrome | |||
| Positive | 1 (1.7) | 1 (1.2) | 0 |
Demographic Data of Patients in Each Group
Before the initiation of chemotherapy, the frequency of fatty liver was 2% (n = 3), all of which were grade I. After chemotherapy, fatty liver was reported in 45.4% of patients without history of fatty liver in their prechemotherapy assessment. Considering the severity of fatty liver after chemotherapy among patients, grade I was the most frequent grade with 70.4%, followed by grades II and III with 28.1% and 1%, respectively (P = 0.0001). In patients with breast cancer, the prevalence of fatty liver before chemotherapy was 3.5%, all were reported grade I. The frequency of fatty liver after chemotherapy in this group of patients significantly increased so that fatty liver was reported in half of the patients (52%); the fatty liver severity after chemotherapy was grade I (29 cases, 64%), grade II (15 cases, 33%), and grade III (one case, 2.2%) (P = 0.0001). Fatty liver was not diagnosed in any gastric cancer patients before chemotherapy. But, after chemotherapy, fatty liver was reported in 38.8% (n = 26). Fatty liver after chemotherapy was grade I (21, 80.7%) and grade II (5, 19.3%), respectively, in gastrointestinal cancer patients while grade III fatty liver was not observed in any patients.
The frequency of fatty liver after chemotherapy was not different between patients in different age groups (P = 0.9). Comparing the prevalence of fatty liver after chemotherapy by sex showed that after chemotherapy, fatty liver was reported in 52.4% of women and 34.7% of men, which showed a significant difference (P = 0.04). Comparing the prevalence of fatty liver occurrence according to pretreatment BMI showed that the prevalence of fatty liver in obese, overweight, normal, and underweight patients was 69.2%, 54.5%, 43%, and 35%, respectively (P = 0.1). There was no correlation between the history of diabetes mellitus and metabolic syndrome and the frequency of fatty liver occurrence after chemotherapy (P = 0.2 and P = 0.4, respectively) (Table 3).
| Before | After | P Value | |
|---|---|---|---|
| Positive | Negative | ||
| All Patients | 0.0001 | ||
| Negative | |||
| N | 80 | 69 | |
| % with FL | 53.7% | 46.3% | |
| % without FL | 98.8% | 97.2% | |
| Positive | |||
| N | 1 | 2 | |
| % with FL | 33.3% | 66.7% | |
| % without FL | 1.2% | 2.8% | |
| Total | |||
| N | 81 | 71 | |
| % with FL | 53.3% | 46.7% | |
| % without FL | 100% | 100% | |
| BC | 0.0001 | ||
| Negative | |||
| N | 39 | 43 | |
| % with FL | 47.6% | 52.4% | |
| % without FL | 97.5% | 95.6% | |
| Positive | |||
| N | 1 | 2 | |
| % with FL | 33.3% | 66.7 | |
| % without FL | 2.5% | 4.4% | |
| Total | |||
| N | 40 | 45 | |
| % with FL | 47.1% | 52.9% | |
| % without FL | 100% | 100% | |
| GIC | n/s | ||
| Negative | |||
| N | 41 | 26 | |
| % with FL | 61.2% | 38.8% | |
| % without FL | 100% | 100% | |
| Positive | |||
| N | 0 | 0 | |
| % with FL | 0% | 0% | |
| % without FL | 0% | 0% | |
| Total | |||
| N | 41 | 26 | |
| % with FL | 61.2% | 38.8% | |
| % without FL | 100% | 100% | |
Relationship Between Chemotherapy and Fatty Liver Incidence
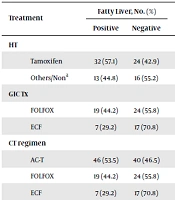
Regarding the effects of tamoxifen on fatty liver occurrence in breast cancer patients undergoing hormone therapy, the results showed that the frequency of fatty liver was 57.1% in tamoxifen users and 44.8% in other patients although the difference was not statistically significant (P = 0.28). The OR of fatty liver was 1.6 for tamoxifen, which means that the risk of developing fatty liver was 1.6 times higher when using tamoxifen. The frequency of fatty liver after chemotherapy was 44.2% (n = 19) in patients with gastrointestinal cancer treated with the FOLFOX regimen and 29.2% (n = 7) in patients treated with the ECF regimen; despite the higher incidence of fatty liver in patients undergoing FOLFOX treatment, the observed difference was not statistically significant (P = 0.26) (Table 4).
| Treatment | Fatty Liver, No. (%) | P Value | |
|---|---|---|---|
| Positive | Negative | ||
| HT | 0.28 | ||
| Tamoxifen | 32 (57.1) | 24 (42.9) | |
| Others/Nona | 13 (44.8) | 16 (55.2) | |
| GIC Tx | 0.26 | ||
| FOLFOX | 19 (44.2) | 24 (55.8) | |
| ECF | 7 (29.2) | 17 (70.8) | |
| CT regimen | 0.09 | ||
| AC-T | 46 (53.5) | 40 (46.5) | |
| FOLFOX | 19 (44.2) | 24 (55.8) | |
| ECF | 7 (29.2) | 17 (70.8) | |
| Drug group | 0.056 | ||
| 5FU-based | 25 (37.9) | 41 (62.1) | |
| ADR-based | 46 (53.5) | 40 (46.5) | |
| Drug group | 0.06 | ||
| Alkylating-based | 64 (50) | 64 (50) | |
| non-Alkylating-based | 7 (29.2) | 17 (70.8) | |
Evaluation and Comparison of the Frequency Distribution of Fatty Liver Occurrence After Chemotherapy in Breast Cancer Patients by the Type of Treatment
5. Discussion
Fatty liver and hepatic steatosis are among the most important chronic injuries following chemotherapy and cytotoxic drugs that are used in the treatment of cancer patients. These hepatic disorders may lead to non-alcoholic fatty liver disease (14). This study was conducted to determine the frequency of fatty liver occurrence in breast and gastrointestinal cancers during and after chemotherapy and to investigate some of its risk factors.
In this regard, the results showed that fatty liver occurrence was significantly associated with chemotherapy such that its frequency increased from 2% to 46.7% after chemotherapy. Similar findings were reported in subgroups of patients with various diseases, showing that the frequency of fatty liver raised from about 4% to nearly 50% in patients with breast cancer and from 0% to around 40% in patients with gastrointestinal cancer. However, the severity of the fatty liver after chemotherapy was often of grade I, and only 1% of patients developed grade III fatty liver after chemotherapy. The only demographic factor that was associated with the occurrence of fatty liver after chemotherapy was gender. The frequency of fatty liver after chemotherapy was significantly higher in women than in men. On the other hand, there was no correlation between the presence of chemotherapy after fatty liver and age, BMI, history of diabetes mellitus, and metabolic syndrome. The highest frequency of fatty liver was observed in patients treated with AC-T, FOLFOX, and ECF with 53.5%, 42.9%, and 29.2%, respectively. The incidence of fatty liver was 57.1% in tamoxifen users. There was no correlation between chemotherapy and hormone therapy and fatty liver incidence.
In the present study, fatty liver was reported in approximately 40% of patients with gastric cancer after chemotherapy. Similar studies reported that chemotherapy was likely to occur in 25 to 90% of gastric cancer patients following chemotherapy (8-10). However, no previous study has reported the evidence of severe fatty liver disease after chemotherapy, which is consistent with our study that detected it in only one percent of patients. Miyake et al. reported hepatic steatosis in 34.9% of patients in the 5FU treatment group, up to 75% of which were mild (8). Hubert et al. (9) evaluated the pathologic changes in normal liver parenchyma during treatment with neoadjuvant chemotherapy among metastatic colon cancer patients with medication regimens mainly containing 5FU, oxaliplatin, and irinotecan by histological evaluation; the most common pathologic finding was liver steatosis, which was present in over 90% of patients although only 26% were moderate to severe. Urdzik et al. (10) study of the fatty liver following chemotherapy by MRI showed that its frequency was 25.7% after chemotherapy, all of which were grade I.
Most studies of fatty liver have been performed using drugs such as 5FU and oxaliplatin because of the importance of remaining the liver parenchyma healthy following metastasectomyin patients with metastatic colorectal cancer with liver involvement. Concerning the effects of chemotherapy on fatty liver in patients with gastrointestinal cancer, as shown also in the present study, most studies have reported a significant increase in fatty liver occurrence after chemotherapy despite the use of different diagnostic approaches (7-10). Fernandez et al. (7) evaluated fatty liver occurrence in patients with colorectal cancer after chemotherapy and the results showed that fatty liver significantly increased after chemotherapy. In another study, Miyake et al. (8) evaluated the effect of oral 5-Fluorouracil administration on fatty liver incidence (CT-based diagnosis); the evaluation of the density of liver fat in CT scan images before and after treatment in colon cancer patients showed that mean Hounsfield units score of liver decreased significantly after treatment although it was significantly close to normal by the end of chemotherapy treatment. Compared to the untreated control group, chemotherapy was associated with an increased incidence of fatty liver (8). Other studies in patients with other organ malignancies treated with drugs such as 5FU also reported similar results. Among them, there is a study by Tsuji and Doyama (15), in which the probability of hepatic steatosis occurrence with S1 drug (containing Tegafur as a 5FU premedication) was assessed by Hansfield liver score and liver-to-spleen ratio in pancreatic cancer patients. The results showed that the ratio of liver-to-spleen decreased after treatment with S1, indicating an increase in hepatic steatosis following administration of this drug (15).
Fewer studies have been performed on the association of fatty liver with chemotherapy in patients with breast cancer who are often treated with drugs such as doxorubicin, cyclophosphamide, and paclitaxel; therefore, studies of patients with different malignancies and similar medications are mentioned. In the present study, more than 50% of breast cancer patients developed fatty liver after chemotherapy. Ahn et al. (16) reported that the frequency of fatty liver incidence was 25.8% in patients with breast cancer undergoing adjuvant chemotherapy with doxorubicin with cyclophosphamide. In another study, Ben‐Yakov et al. (11) examined the incidence of fatty liver in patients with non-Hodgkin's lymphoma treated with regimens containing rituximab, doxorubicin, cyclophosphamide, and prednisolone with or without etoposide, and the results showed that after treatment, up to 92% of the patients developed some degrees of hepatic steatosis.
In addition to chemotherapy, tamoxifen is one of the drugs used in the adjuvant treatment of breast cancer and fatty liver has been reported repeatedly following its consumption. In the present study, fatty liver was higher in patients taking tamoxifen than in other patients although the difference was not significant. Pan et al. (17) compared women with breast cancer treated with tamoxifen during a 26 months’ follow-up assessed by ultrasound alongside liver enzymes for fatty liver, with a control group that was not taking tamoxifen. The results showed that after treatment with tamoxifen, the progression of fatty liver or fatty liver formation was three times higher compared to the control group. After treatment, the frequency of mild fatty liver was 22.9 and 25.6%, moderate fatty liver was 11.4 and 28.6%, and severe fatty liver was 0 and 7.9% in the control and tamoxifen groups, respectively, which indicated an increased risk of developing or worsening fatty liver after the administration of tamoxifen (17). Hong et al. (18) compared the frequency of fatty liver in patients with breast cancer treated with tamoxifen and aromatase inhibitors. Of 328 patients in the study, 62 patients in the tamoxifen group and 41 patients in the aromatase inhibitor group developed fatty liver (P = 0.02). The highest risk of fatty liver disease was during the first two years of treatment. The hazard ratio (HR) for fatty liver in tamoxifen use was 1.61 (P = 0.03), independent of obesity and serum cholesterol levels (18).
In this study, the only factor associated with the occurrence of the fatty liver after chemotherapy was the female gender. The results of various studies on this topic have been heterogeneous although they have often emphasized that demographic factors have the least effect on fatty liver formation. In the study by Tsuji and Doyama (15), the results showed that the demographic characteristics of patients with and without fatty liver were not different. Also, the only factor affecting the occurrence of the fatty liver was the body mass index while chemotherapy regimen and blood biochemical indices had no role in predicting it (10). On the other hand, Hubert et al. study showed that alcohol consumption and diabetes mellitus were associated with fatty liver disease and other demographic factors did not play a role (9). Ben‐Yakov et al. (11) showed the incidence of the fatty liver after chemotherapy was more in patients with higher BMI and pre-treatment history of hyperlipidemia than in other patients.
One of the limitations of the present study was the use of ultrasound as a definitive diagnostic tool for determining the presence or absence of fatty liver. In addition to abdominal ultrasound, diagnostic tools for fatty liver include biopsy, laboratory tests, liver vessels Doppler ultrasound, FibroScan, and computed tomography scans, each with its advantages and disadvantages (19). Taking these into account, as well as incorporating project costs into the decision, the researchers used abdominal ultrasound, which has good sensitivity and relative specificity in the diagnosis of fatty liver. Another limitation of the present study is the low frequency of patients with diabetes mellitus or metabolic syndrome, which makes it difficult to generalize the results of this study to high-risk populations.
In future studies, it is proposed to use other methods, such as laboratory tests or FibroScan, in the evaluation of fatty liver in patients undergoing chemotherapy. It is also suggested that in future studies, patients be followed up after chemotherapy discontinuation until the fatty liver disappearance for other environmental factors affecting the occurrence of fatty liver. In addition, consideration should be given to high-risk populations, such as obese, diabetic, or metabolic syndrome patients, to evaluate the impact of chemotherapy in these populations.
5.1. Conclusions
The results showed that chemotherapy was associated with a significantly increased risk of fatty liver, which was higher in women than in men. The occurrence of the fatty liver following chemotherapy was expected in all cases, regardless of diabetes, metabolic syndrome, BMI, and the age of the patients. It should be noted, however, that the severity of fatty liver in most cases was grade I or at most grade II, indicating that after the discontinuation of chemotherapy, if patients observe healthy behaviors (e.g., a healthy diet and proper physical activity), it can be expected that their fatty liver will be eliminated after some time.
.jpg)