1. Background
Chronic Hepatitis B Virus (HBV) infection has been known as a major public health issue worldwide due to high morbidity and mortality (1). Despite the identification of its pathogenesis and antiviral therapeutic approaches, it accounts for nearly one million deaths per year around the world due to hepatic diseases, including chronic hepatitis, cirrhosis, fibrosis, and Hepatocellular Carcinoma (HCC) (2). Regarding Chronic Hepatitis B (CHB)-infected cases, almost all instructions suggest not only to inhibit HBV replication when the level of alanine aminotransferase (ALT) is near the threshold for initiating treatment but also to monitor the degree of liver fibrosis (3).
Liver fibrosis is a common response to HBV infection, which may result in potentially lethal sequelae and progression of compensated cirrhosis to hepatic decompensation and HCC. Incorrect diagnosis and monitoring of liver fibrosis can lead to progressive inflammation and fibrosis, resulting in liver cirrhosis and even HCC (4). Liver fibrosis degree can significantly affect the prognosis and treatment of chronic liver diseases. Considering the prognosis of hepatic diseases and the association of treatment success with fibrosis degree, the precise evaluation of liver fibrosis degree is highly important in CHB-infected patients (5).
Liver biopsy has been used as a reference to diagnose the liver fibrosis stage. Nonetheless, this technique is invasive, expensive, and associated with complications, sampling errors, and probable risks in clinical use (3, 5). Therefore, the development of non-invasive approaches to evaluate liver fibrosis is recommended for improving the treatment of CHB and for the preparation of the liver biopsy (3, 6).
Currently, the most recommended non-invasive method for this purpose is Liver Stiffness Measurement (LSM) using Transient Elastography (TE), which is a fast, precise, and non-invasive technique for estimating the fibrosis stage among CHB cases. However, this technique is ineffective in diagnosing mild and significant liver fibrosis. Also, several factors, such as ALT flares, cardiac insufficiency, extrahepatic cholestasis, inflammation, obesity, and presence of ascites associated with liver stiffness, can limit the applicability of TE (7-9). In fact, the extensive application of TE in the assessment of all patients with CHC is not realistic, and there is still a need to improve the existing predictive models for staging fibrosis as a complementary approach for TE.
The liver receives blood from both the portal vein and hepatic artery and is modulated by the hepatic arterial blood flow response, as increased portal blood flow may reduce hepatic arterial blood flow, and vice versa (10, 11). Due to liver fibrosis, hepatic arterial blood flow may decrease, leading to a hyperdynamic circulation and increased cardiac output. Since liver fibrosis is a major factor in portal hypertension, liver stiffness can be predicted by the presence of portal hypertension in patients with liver fibrosis (12). In several studies, considering the good correlation between hepatic arterial hypertension and LSM, a good diagnostic performance has been reported for advanced liver fibrosis (13-17). These findings support the hypothesis that hepatic arterial blood flow index (HBI) can be applied in humans and that fibrosis patients show a hepatic arterial blood flow response.
Radiological imaging technologies involving scintigraphy are widely used to provide valuable information for the detection and characterization of liver failure and cirrhosis and identify an appropriate treatment method in clinical practice (18-20). In addition, the use of scintigraphy to study liver perfusion and its changes in liver stiffness has been reported (14, 21). Therefore, Hepatic Arterial Perfusion Scintigraphy (HAPS) facilitates the overall assessment of HBI (hepatic artery blood flow/total hepatic blood flow) and represents the correlation between HBI and liver stiffness to present a diagnostic algorithm for liver fibrosis (22, 23).
2. Objectives
In this study, we aimed to conduct a systematic analysis of the non-invasive diagnostic performance of HBI using HAPS and introduce an improved algorithm to assess liver stiffness and liver fibrosis staging among patients with CHB infection as a complementary approach to TE.
3. Methods
3.1. Subjects
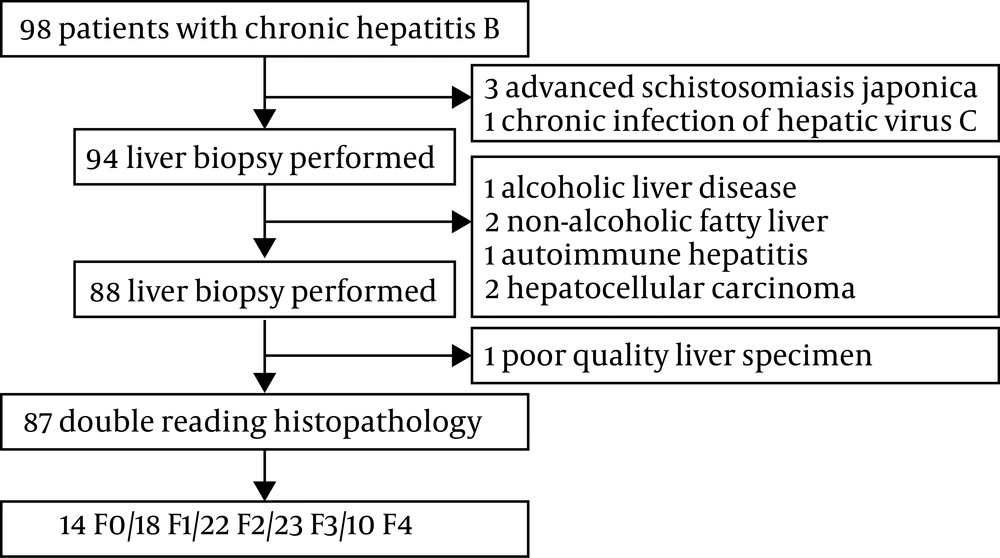
In this research, 98 CHB-infected patients, who were admitted to a hospital affiliated to Jiaxing University in East China between January 2013 and December 2016, were retrospectively recruited. The existence of HBsAg for at least six months was considered as CHB infection. Patients with the following characteristics were excluded from the study: Mixed liver disease; coinfection with HAV, HCV, HDV, or HIV; excessive alcohol intake, combined with renal artery stenosis and renal hypertension; glomerular filtration rate below 60 mL/min; primary liver cancer or other malignant tumors; severe heart, liver, or kidney function disorders; and pregnancy. In total, 87 eligible patients were included in this study, as shown in Figure 1 and anonymously depicted in Table 1. The research was confirmed by the Ethics Committee of the affiliated hospital of Jiaxing University. All participants gave written consent before the study.
| Characteristics | Chronic Hepatitis B (N = 87) |
|---|---|
| Gender (M/F) | 44/43 |
| Age (years) | 53.1 (23.0 - 83.0) |
| ALT (U/L) | 32.0 (12.0 - 483.0) |
| AST (U/L) | 34.0 (16.0 - 237.0) |
| PLT (109 L-1) | 138.5 (24.0 - 315.0) |
| GGT (U/L) | 42.0 (12.0 - 1140.0) |
| BUN (mmol/L) | 4.47 (2.81 - 11.67) |
| ALP (U/L) | 90.0 (45.0 - 645.0) |
| Fibrosis marker tests | |
| HA (ng/mL)/LN (μg/mL)/IV-C (μg/mL)/PCIII (ng/mL) | 107.6 (31.2 - 676.5)/88.2 (24.7 - 146)/62.4 (10 - 217.6)/9.1 (1.5 - 175.1) |
| LSM (kPa) | 11.2 (3.6 - 39.0) |
| APRI | 0.64 (0.19 - 6.58) |
| FIB-4 | 2.57 (0.55 - 21.16) |
| Inflammation stage, No. (%) | |
| A0/A1/A2/A3 | 10 (11.5)/32 (36.8)/35 (40.2)/10 (11.5) |
| Fibrosis stage, No. (%) | |
| F0/F1/F2/F3/F4 | 14 (16.1)/18 (20.7)/22 (25.3)/23 (26.4)/10 (11.5) |
Baseline Characteristics of Patients in This Study
3.2. Liver Histopathology Evaluation
Liver biopsy was performed by qualified histopathologists using 18-gauge biopsy needles (Bard, Covington, GA) under the guidance of ultrasound. All liver biopsy samples were routinely fixed in 10% formalin, followed by embedding in paraffin, slicing into the sections of 4 μm, and staining by hematoxylin-eosin, reticular fiber stain, and Masson's trichrome in each section. Inflammation was graded as A0 - A4 according to an 18-point Histology Activity Index (HAI), while fibrosis was staged F0 - F4 (F0: no fibrosis; F1: fibrosis without septa; F2: portal fibrosis with rare septa; F3: numerous septa but no cirrhosis; and F4: cirrhosis) according to the METAVIR scoring system by two independent, experienced pathologists (Appendix 1 in Supplementary File) (24-26).
3.3. Biochemical Measurements
Clinical laboratory parameters were measured and recorded on the same day as liver biopsy in the same laboratory, according to the producer’s instructions. Blood biochemical parameters, including ALT, aspartate aminotransferase (AST), platelet (PLT), gamma-glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), and alkaline phosphatase (ALP), were tested by the Hitachi 7600 analyzer (Hitachi High-Technologies Corporation, Japan). The HBV serological markers were examined using a chemiluminescence immune analyzer (Abbott Laboratories, Chicago, IL, USA). Moreover, the HBV DNA level was examined using the ABI 7500 real-time PCR system (Applied Biosystems, Foster City, USA) with a lower limit of detection of 1000 IU.mL-1.
3.4. Hepatic Arterial Perfusion Scintigraphy Analysis
Hepatic Arterial Perfusion Scintigraphy (HAPS) with the measurement of HBI was performed using a millennium VG nuclear gamma camera (GE Healthcare, Milwaukee, WI, USA), as previously reported (14, 18, 19). The suggested intravenous dose range of technetium-99m methylene diphosphonate (99mTc-MDP) in an average patient (70 kg) is 370 to 740 MBq (10 - 20 mCi) according to the body mass index. Abdominal images (the liver and kidney) were taken every one second for one minute in a 256 × 256 acquisition matrix according to the described protocol (14). The Area Under the Receiver’s Operating Characteristic (AUROC) curve was applied for measuring the diagnostic value based on regression analysis. By determining the Region of Interest (ROI), HBI was determined as the quotient between the hepatic arterial gradient (G1) and the sum of arterial and portal venous gradients [(G2): HBI= (G1)/(G1 + G2)] in the anterior views of planar images. The observer was unaware of the name and histopathological findings of the cases.
3.5. Statistical Analysis
Student’s t-test or ANOVA was used to analyze the differences between the groups. Pearson’s correlation test was also applied to evaluate the linear relationship between two continuous variables. The AST/PLT Ratio Index (APRI) and the fibrosis index based on 4 factors (FIB-4) were calculated according to similar studies, as follows: APRI = AST (/ULN) × 100/PLT (109 L-1); FIB-4= Age (year)×AST [UL-1]/(PLT [109 L-1]×(ALT [UL-1])1/2). Data were analyzed by SPSS 20.0 at a significance level of less than 0.05. The values are presented as mean ± SD.
4. Results
4.1. Clinical Characteristics of the Study Population
To evaluate the clinical application of HBI in the prediction of liver fibrosis, 98 consecutive CHB patients subjected to TE and biopsy were first enrolled from January 2013 to December 2016. Eighty-seven (88.8%) patients were identified and considered eligible for further analysis. The main characteristics of the subjects are presented in Table 1 and the file appendix 3. As shown in this table, the subjects’ average age was 53.1 years, and men comprised 50.6% of the cases. The mean ALT, AST, PLT, LSM, APRI, and FIB-4 were 32.0, 34.0, 138.5, 11.2, 0.64, and 2.57, respectively. The following fibrosis stages were found: F0 in 14 subjects (16.1%); F1 in 18 subjects (20.7%); F2 in 22 subjects (25.3%); F3 in 23 subjects (26.4%); and F4 in 10 subjects (11.5%).
4.2. Correlation Between Fibrosis Markers and the Degree of Liver Fibrosis
Box plots of HBI, LSM, APRI, and FIB-4 based on liver fibrosis grades assessed through the METAVIR scoring system are presented in Figure 2. Spearman’s correlation coefficient (r) and Eta value for correlations between clinical characteristics and liver fibrosis stages are shown in Supplementary File (Appendix 2). Table 2 presents the correlation between fibrosis markers and the degree of liver fibrosis based on liver fibrosis grades assessed through the METAVIR system. The fibrosis grade showed a positive association with APRI (r = 0.399; Eta value = 0.469; P < 0.0001), FIB-4 (r = 0.523; Eta value = 0.550; P < 0.0001), LSM (r = 0.452; Eta value = 0.471; P < 0.0001), and HBI (r = 0.672; Eta value = 0.763; P < 0.0001), as shown by Spearman and Eta correlation coefficients. The HBI was superior to APRI, FIB-4, and LSM in predicting liver fibrosis, with Spearman’s correlation coefficients of 0.399, 0.523, and 0.452, respectively. Overall, these liver fibrosis markers were well correlated with biopsy scores, especially when excluding cirrhosis and fibrosis.
| R | R Square | Eta Value | Eta Square | |
|---|---|---|---|---|
| APRI | 0.399 | 0.16 | 0.469 | 0.22 |
| FIB-4 | 0.523 | 0.274 | 0.55 | 0.303 |
| LSM | 0.452 | 0.203 | 0.471 | 0.222 |
| HBI | 0.672 | 0.485 | 0.763 | 0.584 |
Correlation Coefficient Between Relevant Fibrosis Markers and Liver Fibrosis Stages
4.3. Comparison of AUROC Between HBI and Other Established Non-Invasive Fibrosis Markers
To further compare the diagnostic performance of the non-invasive fibrosis markers studied, their performance was evaluated concerning diagnostic accuracy and AUROC. For predicting fibrosis and cirrhosis, specificity (Sp), sensitivity (Se), negative predictive value (NPV), and positive predictive value (PPV) were determined. In a cohort of patients with CHB infection, HBI (from 0.33 ± 0.07 to 0.67 ± 0.10 for F1 to F4, respectively) increased as the liver fibrosis stage increased (Appendix 3 in Supplementary File).
For the prediction of significant fibrosis (≥ F2), the AUROC of HBI (0.884; 95% CI: 0.806 - 0.961; P = 0.000) was significantly more than that of LSM (0.807; 95% CI: 0.703 - 0.912; P = 0.000), APRI (0.684; 95% CI: 0.556 - 0.812; P = 0.009), and FIB-4 (0.757; 95% CI: 0.641 - 0.873; P = 0.000) (Table 3). Regarding the diagnostic performance of liver fibrosis for cirrhosis stage, the AUROC (95% CI) was 0.938 (0.880 - 0.995) for HBI, 0.790 (0.645 - 0.934) for LSM, 0.862 (0.770 - 0.954) for APRI, and 0.869 (0.781 - 0.957) for FIB-4. The nomogram showed the good agreement of HBI in predicting the stages of liver fibrosis for CHB patients (Appendix 4A in Supplementary File). Analysis of accuracy showed that the Integrated Discrimination Index (IDI) and the Net Reclassification Index (NRI) were 0.051 and 0.133 for HBI, respectively. Therefore, our results showed a greater potential for HBI in accurately predicting the liver fibrosis stages.
| Fibrosis Stages | Cutoff | AUROC (95% CI) | Se (%) | Sp (%) | PPV (%) | NPV (%) | SE | P Value |
|---|---|---|---|---|---|---|---|---|
| HBI | ||||||||
| ≥ F1 | 0.35 | 0.848 (0.748 - 0.948) | 75.9 | 78.6 | 66.7 | 96.6 | 0.051 | 0.000 |
| ≥ F2 | 0.41 | 0.884 (0.806 - 0.961) | 77.8 | 81.2 | 53.8 | 72.0 | 0.039 | 0.000 |
| ≥ F3 | 0.46 | 0.924 (0.856 - 0.993) | 75.0 | 91.8 | 50.0 | 85.7 | 0.035 | 0.000 |
| F4 | 0.61 | 0.938 (0.880 - 0.995) | 80.0 | 94.1 | 50.0 | 92.6 | 0.029 | 0.000 |
| LSM | ||||||||
| ≥ F1 | 7.30 | 0.757 (0.623 - 0.890) | 88.9 | 43.9 | 66.7 | 90.0 | 0.068 | 0.003 |
| ≥ F2 | 8.90 | 0.807 (0.703 - 0.912) | 83.3 | 62.5 | 50.0 | 89.7 | 0.053 | 0.000 |
| ≥ F3 | 10.50 | 0.822 (0.723 - 0.921) | 91.7 | 68.2 | 33.3 | 92.9 | 0.050 | 0.000 |
| F4 | 14.50 | 0.790 (0.645 - 0.934) | 80.0 | 79.3 | 50.0 | 85.2 | 0.074 | 0.004 |
| APRI | ||||||||
| ≥ F1 | 0.42 | 0.778 (0.627 - 0.929) | 81.5 | 71.4 | 54.5 | 85.2 | 0.077 | 0.001 |
| ≥ F2 | 0.64 | 0.684 (0.556 - 0.812) | 61.1 | 68.7 | 42.9 | 84.6 | 0.065 | 0.009 |
| ≥ F3 | 0.69 | 0.633 (0.490 - 0.775) | 66.7 | 63.6 | 33.3 | 92.9 | 0.073 | 0.072 |
| F4 | 1.22 | 0.862 (0.770 - 0.954) | 80.0 | 75.9 | 33.3 | 85.2 | 0.047 | 0.000 |
| FIB-4 | ||||||||
| ≥ F1 | 1.59 | 0.825 (0.719 - 0.923) | 77.8 | 71.4 | 54.5 | 77.8 | 0.054 | 0.000 |
| ≥ F2 | 2.18 | 0.757 (0.641 - 0.873) | 72.2 | 62.5 | 50.0 | 92.9 | 0.059 | 0.000 |
| ≥ F3 | 3.50 | 0.701 (0.561 - 0.841) | 66.7 | 63.6 | 33.3 | 92.9 | 0.071 | 0.007 |
| F4 | 4.50 | 0.869 (0.781 - 0.957) | 80.0 | 82.8 | 60.0 | 92.6 | 0.045 | 0.000 |
Summary Performance Characteristics of Non-invasive Markers for the Diagnosis of Liver Fibrosis Stages
4.4. Construction of a Novel Evaluation Algorithm for Liver Fibrosis Stages
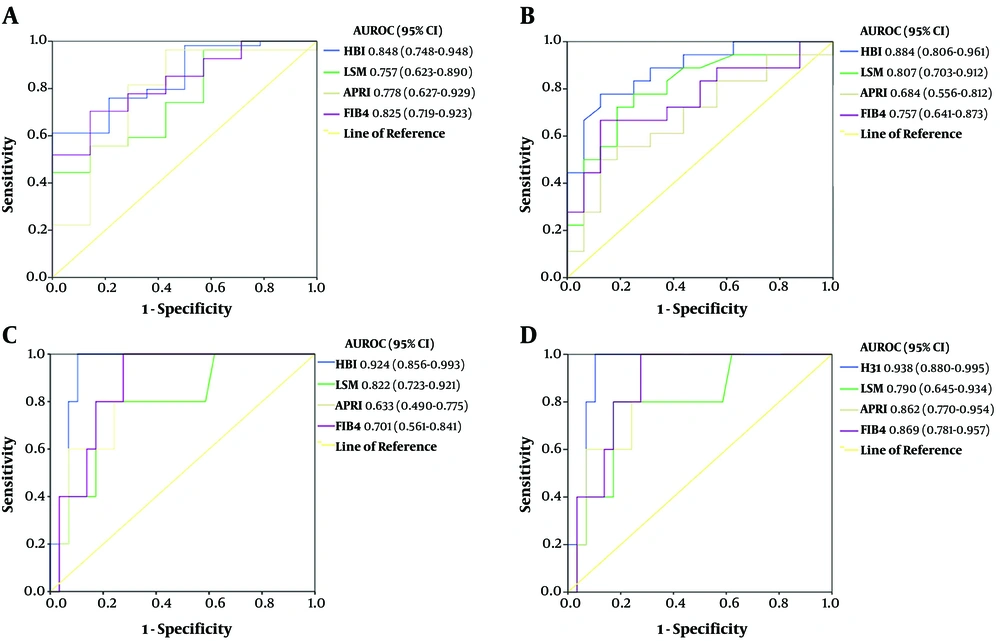
Since HBI showed better diagnostic performance than did other tests in distinguishing liver fibrosis stage, we plotted AUROC curves for ≥ F1 (Figure 3A), ≥ F2 (Figure 3B), ≥ F3 (Figure 3C), and F4 (Figure 3D) stages in 87 CHB patients from the validation cohort (appendix 5 and 22). According to different METAVIR fibrosis scores, the AUROC of HBI was 0.848 (95% CI: 0.748 - 0.948; P = 0.000) in patients with fibrosis stage ≥ F1, 0.884 (95% CI: 0.806 - 0.961; P = 0.000) in cases with fibrosis stage ≥ F2, 0.924 (95% CI: 0.856 - 0.993; P = 0.000) in cases with fibrosis stage ≥ F3, and 0.938 (95% CI: 0.880 - 0.995; P = 0.000) in cases with fibrosis stage F4 (Figure 3). A significant correlation was observed between the HBI values and liver fibrosis stage in 87 CHB patients. These results clearly indicated that the performance of HBI was as good as the performance of APRI, FIB-4, and LSM in predicting the degree of liver fibrosis. Thus, a diagnostic algorithm for HBI was proposed to assess liver fibrosis, according to HAPS.
The AUROC curves of HBI, LSM, APRI, and FIB-4 in predicting different levels of fibrosis in CHC patients. (A) ROC curve for liver fibrosis ≥ F1 stage. (B) ROC curve for significant liver fibrosis (≥ F2). (C) ROC curve for advanced liver fibrosis (≥ F3). (D) ROC curve for liver cirrhosis (F4). AUROC: area under the receiver's operating characteristic curve; 95% CI: 95% confidence interval.
5. Discussion
Hepatitis B virus infection is still an important public health issue in the world (2, 27). Since chronic HBV patients with liver fibrosis account for a significant proportion of HCC cases, previous studies have confirmed that liver cirrhosis (F4 stage) is associated with the increased risk of developing HCC, unlike other liver fibrosis stages (11, 28-30). While the existing literature has mostly focused on the adverse effects and costs of current antiviral therapies, little is known about the degree of liver fibrosis to make more informed treatment decisions. Therefore, the precise evaluation of liver fibrosis seems essential to identify susceptible cases to severe diseases and make appropriate therapeutic decisions for CHB-infected patients (31).
Liver biopsy is still the reference standard for the diagnosis of cirrhosis and staging of liver fibrosis in CHB patients; however, it has certain limitations as an invasive test with some serious health risks (8). Therefore, extensive studies have considered evaluating non-invasive techniques regarding liver fibrosis, including blood biomarkers, such as APRI and FIB-4, and imaging techniques, such as TE via ultrasound, optical digital analysis of CT images, and magnetic resonance (MR)-based techniques (32). On the other hand, the limitations of non-invasive techniques include body mass index, steatosis, sampling variability, high cost, low sensitivity or specificity, and lack of standardized cutoff points for the assessment of fibrosis stage, as mentioned in previous studies. To overcome the limitations of these methods, we developed a diagnostic algorithm for the prediction of liver fibrosis, using HBI-based HAPS with Tc-99m MDP as a potential alternative.
In this study, a comparison was made between the diagnostic value of HBI and other non-invasive methods, such as APRI, FIB-4, and LSM, in the assessment of liver fibrosis in 87 CHB patients. We established a new algorithm to assess liver fibrosis, which was more effective than other ones. Spearman’s rank correlation coefficient and Eta value of HBI versus the degree of liver fibrosis, according to the METAVIR scoring system, were 0.672 and 0.763 (P < 0.001), respectively, which confirmed HBI changes associated with fibrosis stage. There is an association between the degree of liver fibrosis and portal pressure, evidenced by comparing histological alterations in liver biopsy and HAPS (9, 33). In physiological conditions, the portal vein and hepatic artery provide two-thirds and one-third of the blood flow for the liver, respectively. Nonetheless, in liver fibrosis and cirrhosis, the liver counterbalances this event through an increase in the hepatic arterial blood flow for maintaining the same total perfusion. Increased hepatic arterial blood flow is correlated with elevated elastometry in cirrhotic cases in response to meal ingestion (17, 32-34), which can explain a remarkable increase in HBI with liver fibrosis and a moderate reduction in total perfusion with fibrosis grades.
The performance of non-invasive diagnostic methods can be evaluated by the calculation of AUROC, with liver biopsy as the reference standard. The AUROC has been measured in all selected studies, and the standard error can be calculated or estimated based on the existing information, particularly at a 95% confidence interval (CI). The random-effects model indicated the dissimilarity of the studies to analyze the overall efficacy of non-invasive tests for liver fibrosis in different studies (11, 35).
In the present study, HBI showed better performance in differentiating liver fibrosis stages, with AUROC of 0.848 (95% CI: 0.748 - 0.948) for fibrosis stage ≥ F1, 0.884 (95% CI: 0.806 - 0.961) for fibrosis stage ≥ F2, 0.924 (95% CI: 0.856 - 0.993) for fibrosis stage ≥ F3, and 0.938 (95% CI: 0.880 - 0.995) for fibrosis stage F4. With significant fibrosis defined as ≥ F2, the AUROC of HBI (0.884; 95% CI: 0.806 - 0.961; P = 0.000) was greater than that of LSM (0.807; 95% CI: 0.703 - 0.912; P = 0.000), APRI (0.684; 95% CI: 0.556 - 0.812; P = 0.009), and FIB-4 (0.757; 95% CI: 0.641 - 0.873; P = 0.000) regarding the diagnostic performance of liver fibrosis. Overall, the AUROC values of 100%, over 90%, and more than 80% indicate the perfect, excellent, and good diagnostic tool, respectively (11). Accordingly, HBI is an excellent tool to confirm liver fibrosis when other clinical signs and evaluations are non-decisive.
Non-invasive methods of liver fibrosis can be divided into two categories: The physical approach based on LSM and the biological approach according to the quantification of biomarkers in blood specimens, involved in the molecular pathogenesis of fibrosis (e.g., APRI and FIB-4). The physical approach is related to a genuine and intrinsic physical feature of liver parenchyma, while the biological approach indicates different, not strictly liver-specific clinical and serum factors, correlated with fibrosis grade (35, 36). However, these two categories are limited by the inherent characteristics of liver fibrosis. In clinical practice, TE is not accurate (specificity < 60%) to identify cases with esophageal varices and non-alcoholic fatty liver disease. Furthermore, a recent report suggested that the performance of blood biomarkers, including APRI and FIB-4, to identify and exclude advanced fibrosis may be different during life, as young adults are found with lower accuracy and older adults with a lower specificity.
According to direct comparisons based on the AUROC in CHB patients (Figure 3), HBI performed substantially better than APRI and FIB-4 in the diagnosis of fibrosis and cirrhosis and moderately better than LSM to diagnose fibrosis. Differences between HBI and other blood tests or LSM were relatively small, particularly in the significant fibrosis stage. Based on these results, by using optimal cutoff points as standards, the sensitivity and specificity of HBI were found to be similar in predicting similar stages of fibrosis, whereas those of LSM, APRI, and FIB-4 were slightly irregular to predict similar stages of fibrosis; thus, HBI was significantly associated with the degree of liver fibrosis CHB patients.
Some limitations of this study should be acknowledged. First, this retrospective study was validated in a relatively small sample size of CHB patients. Second, using liver biopsy as a reference standard to evaluate liver stages has methodological flaws, which may influence the performance of the conducted tests. Third, other clinical confounding factors, such as splenomegaly and esophageal varices, may affect liver fibrosis, and these factors need to be considered in future studies.
5.1. Conclusion
We applied the HAPS method, followed by AUROC measurement, to evaluate the association of HBI with the degree of liver fibrosis in CHB patients. The results suggested the potential application of HBI as an accurate and inexpensive alternative for the diagnosis of liver fibrosis stage and guiding therapy in CHB patients. The current study was the first to report using HBI for the detection of liver fibrosis stage in CHB patients. This study not only confirmed earlier findings, showing that scintigraphic evaluation of hepatic blood flow is possible through determining HBI (ratio of hepatic artery flow to total hepatic flow), but also provided new information about a novel, reliable, and non-invasive technique to assess liver fibrosis to facilitate further in-depth clinical applications. The proposed method also is possibly a promising nuclear medicine method to predict liver fibrosis in CHB patients regarding preliminary results.