1. Introduction
The liver plays an important role in metabolism of carbohydrates, proteins, lipids, drugs, and toxins.
The slow and long-term process of liver disease results in progressive increase in number of patients with hepatic disorders. Chronic liver disease includes a wide range of liver pathologies from inflammation to cirrhosis. Cirrhosis is characterized by expansion of regenerative nodules and fibrous bands in response to chronic liver injury, leading to end-stage liver disease. Special attention is needed to choose the best matching pharmacokinetic and pharmacodynamic treatments in patients with liver disease (1).
Dexmedetomidine (DEX) is the new α2-adrenoreceptor agonist that has a complete metabolism in the liver and almost complete excretion in urine (2). This drug has a fast onset of action and minimal side effects. It also has a synergistic effect when combined with most anesthetic drugs. Its side effects include bradycardia, vasoconstriction, and mild respiratory depression (3). Additionally, DEX is also used as an anesthetic drug and has sedative, analgesic and anti-inflammatory effects (4). It can be administered with both intravenous and intranasal methods, and the studies have shown that in addition to its pharmacodynamic and pharmacokinetic features, its analgesic and sedative effects are the same in both methods (5). The DEX dosage in patients with liver failure needs to be adjusted (6). It also needs to be adjusted in patients with obstructive jaundice due to its decreased volume of distribution (7).
Lower doses of DEX have protective effect while high doses (10 µg/kg) have negative effects on the liver tissue which can be minimized by simultaneous administration of 100 mg/kg of vitamin C (8). In patients with irritable bowel disease (IBD), the extra-intestinal manifestations may include liver inflammation. In animal models of IBD, DEX prescription reduces ultra-structural and histopathological damage of liver (9). Administration of DEX exerted protective effects against hepatic ischemia-reperfusion injury (IRI) in adult living donor liver transplantation. It results in suppression of ICAM-2, improved scores of histopathalogic assessment, and augmentation of live function tests after surgery (10). Patients with liver transplantation, have a high risk of post-surgery delirium. Administration of DEX for more than 3 days with cycling may be useful in delirium prevention in these patients (11). In children undergoing liver transplantation with post-surgery myocardial or brain injury, DEX can be used to reduce the injury (12, 13). In patients with mild liver dysfunction undergoing laparotomy, DEX is used in combination with propofol and remifentanil for general anesthesia (14). In cirrhotic patients, DEX usage as an anesthetic drug results in improvement of dynamic stability, reduction in stress responnse, and reduction of inflammation rate, without having adverse effect on immunologic functions, which has a significant clinical value (15).
2. Objectives
Considering the mentioned studies and to the best of our knowledge, no systematic review has been conducted in this field. In this respect, our aim in this study is to conduct a systematic review of DEX effects in patients with liver disorders.
3. Methods
This systematic review and meta-analysis follows the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist.
3.1. Literature Search
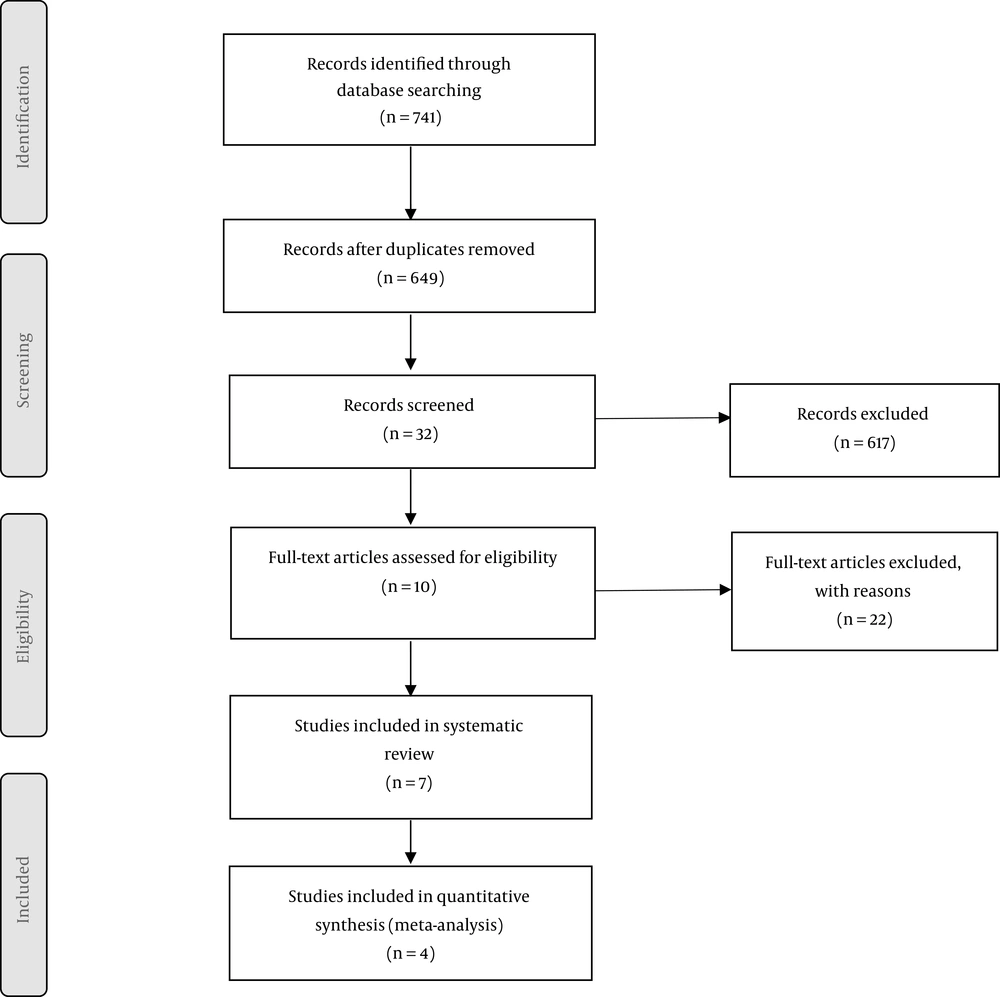
Embase, PubMed, Ovid, Scopus, ProQuest, Web of Science, The Cochrane Libraries and Google Scholar were searched to identify studies that reported the results of dexmedetomidine administration on any form of hepatic injury like hepatic reperfusion injury. Using the Medical Subject Headings (MeSH) and Emtree keywords including “Liver Transplantation”, “Liver Diseases”, “Chronic Hepatitis”, “Cirrhosis”, “Hepatectomy”, “Liver Failure”, “Dexmedetomidine” and combining them with Boolean Operators, studies which were published in English from January 1980 to June 2019 were investigated and other sources including grey literature and articles presented in congresses were also searched. Additional information about the search strategy is presented in the PRISMA flow diagram in Figure 1.
3.2. Study Selection
The inclusion criteria for this study were as follows:
1- Randomized control trials (RCTs) investigating the role of dexmedetomidine in hepatic patients
2- Articles published in English from January 1980 to June 2019
3- Articles with access to their full texts
The exclusion criteria were considered to be as follows:
1- Non-randomized controlled trials
2- Unavailability of the full text of the articles
The results were imported to the Endnote X8 software to remove the duplicates and organize the studies.
3.3. Methodological Quality
Assessment of methodological quality and the risk of bias of enrolled randomized controlled trials were performed using Cochrane checklist to evaluate the randomization sequence, allocation concealment, determination of whether blinding was implemented for participants or outcome assessors, and evidence of selective reporting or other notable biases. Accordingly, articles were categorized into 3 groups: “low risk of bias/L” “high risk of biases/H” or “unclear risk of bias/U”.
3.4. Data Extraction
Two independent reviewers, H.S and K.SH, selected the studies following three steps; first title of all the studies was reviewed, then abstract and full text of the articles was investigated and finally, risk of bias was evaluated to exclude all the irrelevant studies. Any disagreement between reviewers was resolved by consulting with a third evaluator, MG.
Extracted data included first author’s name, year of publication, type of the study, sample size, mean age, weight, sex of the participants, baseline MAP, post-operative MAP, dosage of the drug, condition, effects and adverse effects of the drug.
3.5. Data Analysis
Statistical analysis was performed using CMA version 0.3. Assessment of heterogeneity between studies was done by application of Cochrane’s Q statistic (heterogeneity < 0.10 suggesting statistical significance) and the I2 statistic. I2 < 50% was considered to show no statistical heterogeneity and fixed-effect model was used, on the other hand, I2 ≥ 50% or P value < 0.05 indicated significant heterogeneity, therefore required a random effect model for analysis. P value < 0.05 was considered to be statistically significant.
4. Results
4.1. Search Results and Study Characteristics
In the systematic search, 741 articles were identified. Among them, 92 articles were duplicate and 617 were excluded after reviewing the titles and abstracts. After screening the full text of the articles, 22 articles were excluded. Finally, seven studies conforming to the inclusion criteria entered this systematic review. The flowchart for the articles identified and entered into the study is shown in Figure 1. The characteristics of the included studies are provided in Table 1.
| First Author | Year | Country | Type of Study | Sample Size | Mean Age | Weight | Gender (Male/Female) | Dosage | Condition | Adverse Effects | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hControlh | Treatmenth | Control | Treatment | Control | Treatment | Control | Treatment | |||||||
| Wang (16) | 2014 | China | RCT | 22 | 22 | 58.4± 10.3 | 60.9 ± 9.6 | 16.6 | 17.5 | 1 - 0.3 mg kg21 | Elective hepatectomy with inflow occlusion | |||
| Wang (15) | 2018 | China | RCT | 47 | 47 | 46.85 | 46.56 | 26.21 | 28.19 | 1.0 - 0.5 µg/kg | Liver Cirrhosis | Postoperative agitation | ||
| Fayed (10) | 2016 | Egypt | RCT | 20 | 20 | 52.47 ± 5.8 | 50.6 ± 7.5 | 18.2 | 17.3 | 0.8 lg/kg/h | Hepatic ischemial reperfusion injury (IRI) | |||
| Sayed (17) | 2016 | Egypt | RCT | 20 | 20 | 44.8 | 43.3 | 81.3 ± 9.14 | 76.90 ± 4.39 | 16.4 | 14.6 | 0.2-0.7 µg/kg/h | During living donors liver transplantation (LDLT) on the general anesthetic requirements, hemodynamics, oxygen consumption (VO2), and CO2 production (VCO2) | infusion of DEX as an adjuvant in general anesthesia causes decreased requirement of Des and fentanyl in patients undergoing ALDLT without compromising adequate depth of anesthesia |
| Rodrigues (18) | 1997 | USA | In vitro | 5 | 0.01 - 4.0 µM | Interaction of dexmedetomidine with human liver microsomal cytochrome P4502D6 (CYP2D6) | ||||||||
| Feng (19) | China | In vitro | 44 | 1 µM | Hepatic ischemia-reperfusion injury | |||||||||
| Zhu (20) | 2018 | China | In vitro | 5 µM | Ischemic liver injury | |||||||||
4.2. Participant Characteristics
In this study, 4 studies were finally entered into a meta-analysis. In the selected studies 218 patients in the both control and intervention groups were studied. The mean ± standard deviation of the participants’ age was 48.04 ± 3.97 in the control group and 46.82 ± 3.65 in the intervention group.
4.3. Meta-Analysis Results
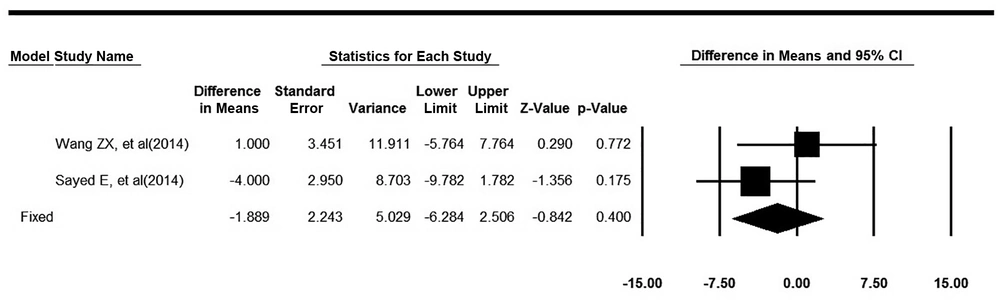
The mean of MAP for the treated patients before and after the intervention was found from the studies. The changes in MAP were calculated in two groups and finally, the changes were combined using the meta-analysis. In this meta-analysis, heterogeneity of studies was not significant. (Q = 1.21, P value = 0.27, I2 = 17.64). Based on the fixed effect model, MAP changes in the intervention group were 1.89 units less than the control group, which was not statistically significant (pooled mean difference = -1.89, 95% CI: -6.28 to 2.5, P value = 0.39). The forest plot related to the integration of the results is shown in Figure 2.
5. Discussion
The present study is a systematic review assessing the effect of DEX in hepatic patients. DEX is widely used as an anesthetic drug for hemodynamic stability during surgery and it has been used as an analgesic agent in surgical procedures in recent years (21). Research in rats with liver, kidney, or lung damage have shown the protective effects of DEX against the damage of these organs (22-24). Other studies in rats showed that DEX reduced plasma levels of MDA and catecholamine (25, 26).
Studies have shown that DEX’s potential mechanism for re-healing of IRI is due to the anti-inflammatory effects of this drug (27). Wang et al. showed that preoperative injection of DEX in patients undergoing elective hepatectomy with inflow occlusion has a potential protective effect on the intestine and liver. During hepatectomy with inflow occlusion by Pringle maneuver, hepatic IRI can result in systemic responses and release of harmful substances in two phases: in the initial phase (in the first 2 hours of hepatic IRI), activation of the liver Koopfer cells and release of oxidative substances occur which causes necrosis and apoptosis in the liver; in the second phase (in the next 6 hours or more), there is an accumulation of neutrophils and inflammatory responses that can damage other organs such as the intestines. Although the mechanism of protective effects of DEX is not yet fully understood, it may play a role in decreasing the plasma levels of catecholamines (16). Wang L. determined that DEX in patients with multiple cirrhosis could improve the clinical status of patients during and after anesthesia. This drug is the selective agonist of α receptor and it inhibits multiple stress responses, thus stabilizes the hemodynamic status (HR, SpO2, PETCO2), minimizes MAP and VAS, and can lower the blood pressure during operation in this group of patients. It induces sedation and analgesia through its effect on potassium channels as well as α2 receptors of locus coeruleus and inhibition of histamine release during and after operation. In addition, DEX reduces the serum levels of aldosterone, cortisol and ACTH during anesthesia and surgery, and in fact has a unique anti-damage effect. Furthermore, DEX, by inhibiting the calcium influx in nerve endings, reduces body temperature, decreases shivering and reduces agitation after surgery. It applies its anti-inflammatory effects by lowering the serum levels of catecholamines and releasing IL-10 and TNF-α (15). Fayed et al. (10)found that DEX injection during anesthesia and after the completion of surgery for patients undergoing liver transplantation (LD Liver Transplantation) has a protective effect on hepatic ischemia-reperfusion injury (IRI), reduces the serum levels of lactate by facilitating the reperfusion of the transplanted tissue, facilitates the weaning and extubation of these patients and reduces their length of stay in ICU. In addition to the mechanisms mentioned in previous studies, other mechanisms of this drug in reducing the tissue ischemia are as follows:
1- Reduces the level of nitric oxide synthesis.
2- Suppresses the secretion of adrenaline and thereby reduces tissue ischemia.
3- Stimulation of the α2-adrenergic receptor which reduces intestinal and myocardial ischemia.
4- A dose of 0.8 µg/kg/h DEX reduces the concentration of isoflurane, inhaled doses of fluoride and reduces fentanyl consumption during surgery.
5- Reduces the number of neutrophils and subsequently reduces endothelin-1 and reduces protease by reducing the level of ICAM-1 (an important molecule in the adhesion of leukocytes during the migration of leukocytes to the inflammation site), which protects organs like intestine and kidney from ischemia while protecting the transplanted organ [10.
Sayed and Yassen showed that DEX can be considered as an adjunctive therapy and have an effective role in stabilizing the hemodynamic symptoms of patients, reducing the dosage of inhalational desflurane and fentanyl consumption without affecting the depth of anesthesia, reducing oxygen consumption and reducing production of CO2. Due to the sympatholytic effect of DEX, the sympathoadrenal response to tracheal intubation of these patients is reduced and intubation is facilitated. Because of its analgesic effect, it provides better analgesia during and after surgery, reduces HR and MAP during intubation and during anesthesia due to reduced stress response, and therefore is effective in stabilizing hemodynamic symptoms in patients. It decreases oxygen consumption throughout the tissues especially the transplanted tissue by a reduction in sympathetic activity and subsequent reduction of total body metabolism, and due to its antinociceptive effect and indirect effect on sedation and neuromuscular block (17).
5.1. Conclusions
Injection of DEX prior to anesthesia potentially had a protective effect on liver and intestinal function during hepatectomy with vascular occlusion. While DEX injection through anesthesia in cirrhotic patients stabilized hemodynamic symptoms and reduced the stress response, it also reduced the level of inflammation without affecting the function of the immune system. Reducing the consumption of fentanyl and desflurane during anesthesia, DEX injection through anesthesia in patients who required liver transplants is effective in stabilizing hemodynamic symptoms, reducing tissue ischemia, and improving liver function.