1. Background
Metabolic syndrome (MetS) is a complex clustering cardiovascular risk factors, including abdominal obesity, hyperglycemia, hyperlipidemia, hypertension (HTN), and decreased high-density lipoprotein (HDL), leading to cardiovascular disease (CVD), diabetes mellitus (DM), and chronic kidney disease (CKD) (1-4). MetS and other cardiometabolic disorders have become major public health concerns with an increasing prevalence worldwide (5, 6).
Liver Function tests (LFTs) typically include serum concentration of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin (TBIL), and lactate dehydrogenase (LDH) (7, 8). These tests can be helpful in the evaluation and treatment of patients with hepatic dysfunction; however, particular attention has recently paid on the role of LFT in predicting cardiometabolic disorders (8). In this regard, several studies have investigated the association between LFT, particularly ALT, AST, and GGT, and MetS and DM, of which some studies have reported positive associations between the enzymes and MetS (9-11) and DM (12-14). A limited number of studies have investigated the association between LFT and CKD; however, most of them have reported lower levels of liver enzymes in CKD patients (15-17). The association between ALP or LDH and cardio-metabolic disorders has been less documented. Notably, the levels of aminotransferases have been shown associated with cardiometabolic disorders not only in elevated levels but also within their normal ranges (9, 18, 19).
Since early detection of MetS and other cardiometabolic disorders is of great importance, finding a clear association between these events and LFT may be helpful for the earlier prognosis of metabolic abnormalities.
2. Objectives
This cross-sectional, population-based study was conducted to assess the correlations between LFTs (including ALT, AST, ALT/AST ratio, ALP, GGT, and LDH) and cardiometabolic disorders (including MetS, HTN, DM, and CKD) among an Iranian population. Also, we analyzed the subjects with liver enzyme levels within the normal range to determine possible predictive values of LFTs with respect to the risk of cardiometabolic disorders.
3. Methods
3.1. Study Population
This cross-sectional study was conducted using the Tehran Lipid and Glucose Study (TLGS) data. Briefly, TLGS is a cohort population-based study on 15,005 residents of district 13 in Tehran, Iran (20). The first phase of the TLGS was initiated from 1999, and data recollection is designed to be in 3-year intervals (21). We recruited 1139 adults from both genders, who had participated in the sixth examination of the TLGS (2014 - 2017), with complete data in terms of LFTs, as well as demographic, anthropometric, and biochemical measurements.
3.2. Anthropometric and Demographic Measurements
The body weight of the participants was measured using digital scales (Seca, Hamburg, Germany), while they were minimally clothed and without shoes. Their height, in a standing position and without shoes, was also measured using a tape meter. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (m2). Also, waist circumference (WC) was measured using a tape meter, while the subjects were lightly clothed.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by a standard mercury sphygmomanometer (22). In the beginning, the participants were in a sitting position. After a 15-minute rest, two measurements of blood pressure were taken with at least a 30-second interval, and their mean was considered as the participant’s blood pressure.
3.3. Biochemical Measures
Blood samples were taken between 7:00 AM and 9:00 AM, after 12 - 14 h of fasting. Fasting plasma glucose (FPG) and 2-hour post-challenge plasma glucose (2h-PCPG) were assayed using glucose oxidase. Serum triglyceride (TG) levels were assayed by enzymatic colorimetric method using glycerol phosphate oxidase. High-density lipoprotein-cholesterol (HDL-C) was measured by the homogenous method (HDLC Immuno FS). Serum liver enzymes were assayed using enzymatic colorimetric methods. All blood analyses were done using Pars Azmoon kits (Pars Azmoon Inc., Tehran, Iran) and a Selectra 2 auto-analyzer (Vital Scientific, Spankeren, The Netherlands) at the research laboratory of the TLGS. Serum creatinine levels were measured by the kinetic colorimetric Jaffe method. Both inter- and intra-assay coefficients of variations (CVs) were < 5%.
3.4. Definition of Terms
MetS components were defined according to the National Cholesterol Education Program (NCEP)-ATP III diagnostic criteria (4). For WC, we used cutoff points for Iranian adults (23). MetS was defined as having at least 3 of the metabolic abnormalities: (1) hyperglycemia as FPG ≥ 100 mg/dL (5.6 mmol/L) or drug treatment for impaired fasting glucose; (2) hypertriglyceridemia as serum TG ≥ 150 mg/dL (1.69 mmol/L) or drug treatment; (3) low HDL-c as serum HDL-c < 40 mg/dL (1.04 mmol/L) for men and < 50 mg/dL (1.29 mmol/L) for women or drug treatment; (4) HTN as blood pressure ≥ 130/85 mmHg or drug treatment for HTN, and (5) abdominal obesity as WC ≥ 95 cm for both genders.
A participant who met at least one of these criteria was considered as a patient with DM: FPG ≥ 126 mg/dL, 2 h-PCPG ≥ 200 mg/dL, or taking anti-diabetic medications (24).
HTN was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or taking blood pressure-lowering medications (25).
CKD was defined as an estimated GFR (eGFR) of less than 60 mL/min per 1.73 m2 (26). eGFR was calculated by the CKD-EPI creatinine equation, developed by the chronic kidney disease epidemiology collaboration:
In this equation, Scr is serum creatinine in mg/dL; κ is 0.7 and 0.9 for men and women, respectively, α is -0.329 and -0.411 for men and women, respectively; min indicates the minimum of Scr/κ or 1, and max represents the maximum of Scr/κ or 1 (27).
3.5. Ethical Consideration
Written informed consent was obtained from all participants, and the study protocol was approved by the ethics research council of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran.
3.6. Statistical Analyses
Mean, and standard deviation (SD) values, and frequency (%) of general characteristics of participants, as well as mean and SD values of liver enzymes were compared across cardiometabolic disorders as dichotomous variables (yes/no), using independent sample t-test or chi-square test, for dichotomous and continues variables, respectively.
To estimate the odds ratio (OR) of cardiometabolic disorders in each tertile category of LFT (ALT, AST, ALT/AST ratio, GGT, ALP, and LDH), the multivariable logistic regression models were used. Potential confounding variables were entered into the univariate models to determine confounders. Variables with PE < 0.2 were selected as confounders. Finally, confounders adjusted in models included sex (male or female), age (year), and BMI (kg/m2).
Finally, logistic regression analysis was conducted to evaluate the presence of MetS, DM, HTN, and CKD with respect to LFT within normal ranges. Due to the lack of any valid reference normal ranges for other liver enzymes in the Iranian population, only ALT and AST were included. According to an Iranian population-based study, reference normal ranges for ALT were considered < 40 and < 34 U/L in men and women, respectively, and < 34 U/L in both gender for AST (28).
All statistical analyses were performed using the Statistical Package for Social Science (version 20; IBM Corp., Armonk, NY, USA). P values < 0.05 were considered significant.
4. Results
The mean age (± SD) of participants was 48.2 ± 17.1 years, and 42.3% of the participants were men. General characteristics of the participants, as well as LFT values in healthy participants and the subjects who had one of the four cardiometabolic disorders, are shown in Table 1. Compared with participants who had no cardiometabolic disorders, subjects with MetS, DM, HTN, or CKD were more likely to be older and had higher BMI, WC, SBP, and DBP. Mean levels of ALT, AST, ALT/AST, GGT, ALP, and LDH were significantly higher in subjects with MetS compared with those without MetS. DM was significantly accompanied by higher levels of GGT and ALP. Subjects with HTN had significantly higher levels of AST, GGT, ALP, and LDH compared with participants without HTN. Moreover, the subjects with CKD were found with significantly higher levels of ALT, ALT/AST ratio, ALP, and LDH than those without CKD.
| Variables | Metabolic Syndrome | Diabetes | Hypertension | Chronic Kidney Disease | ||||
|---|---|---|---|---|---|---|---|---|
| Yes (N = 378) | No (N = 641) | Yes (N = 186) | No (N = 653) | Yes (N = 418) | No (N = 708) | Yes (N = 233) | No (N = 906) | |
| Age, y | 54.0 ± 14.37 | 41.69 ± 14.92b | 62.8 ± 12.50 | 44.17 ± 14.51b | 62.4 ± 14.93 | 42.06 ± 14.16b | 67.8 ± 11.73 | 43.00 ± 14.42b |
| Female, % | 49.3 | 59.7b | 62.6 | 56.4 | 58.4 | 57.6 | 70.7 | 54.5b |
| BMI, kg/m2 | 30.71 ± 5.60 | 26.11 ± 4.45b | 30.39 ± 5.29 | 27.52 ± 4.93b | 30.27 ± 5.33 | 27.02 ± 5.17b | 29.12 ± 4.68 | 27.58 ± 5.51b |
| Waist circumference, cm | 101 ± 9.94 | 87.8 ± 11.44b | 101 ± 11.14 | 92.0 ± 12.35b | 100 ± 11.28 | 90.4 ± 12.20b | 98.3 ± 11.21 | 91.8 ± 12.80b |
| SBP, mmHg | 124 ± 17.82 | 108 ± 15.35b | 127 ± 18.78 | 113 ± 17.17b | 134 ± 19.89 | 108 ± 12.51b | 127 ± 21.70 | 113 ± 17.13b |
| DBP, mmHg | 80.7 ± 10.13 | 73.4 ± 9.02b | 77.8 ± 11.30 | 75.4 ± 10.11b | 83.2 ± 12.59 | 73.4 ± 8.04b | 77.2 ± 11.46 | 75.9 ± 10.26b |
| ALT, U/L | 18.33 ± 13.93 | 14.11 ± 12.17b | 14.81 ± 10.15 | 14.46 ± 11.39 | 15.32 ± 13.10 | 15.40 ± 12.71 | 12.19 ± 9.04 | 16.21 ± 13.52b |
| AST, U/L | 22.64 ± 10.67 | 20.34 ± 13.10b | 20.95 ± 8.66 | 21.07 ± 12.53 | 22.10 ± 15.96 | 20.44 ± 9.70b | 21.07 ± 8.39 | 20.90 ± 12.69 |
| ALT/AST | 0.81 ± 0.44 | 0.68 ± 0.37b | 0.71 ± 0.35 | 0.69 ± 0.39 | 0.70 ± 0.41 | 0.73 ± 0.39 | 0.57 ± 0.30 | 0.76 ± 0.41b |
| GGT, U/L | 27.09 ± 22.94 | 20.23 ± 27.38b | 26.53 ± 18.70 | 22.09 ± 29.34b | 26.53 ± 24.46 | 21.11 ± 25.59b | 23.41 ± 20.45 | 22.54 ± 26.51 |
| ALP, U/L | 195 ± 63.1 | 176 ± 83.2b | 208 ± 66.4 | 178 ± 83.7b | 204 ± 62.6 | 178 ± 79.5b | 198 ± 72.8 | 182 ± 76.2b |
| LDH, U/L | 312 ± 99.6 | 289 ± 77.7b | 306 ± 100 | 293 ± 82.0 | 316 ± 101 | 290 ± 77.1b | 326 ± 103 | 292 ± 80.9b |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; DBP, diastolic blood pressure; GGT, gamma-glutamyl transpeptidase; LDH, lactate dehydrogenase, SBP, systolic blood pressure; WC, waist circumference.
aValues are expressed as mean ± SD.
bP value < 0.05.
The ORs (with 95% CI) of MetS across tertiles categories of LFT are shown in Table 2. In multivariable-adjusted models, subjects in the third tertile of ALT, AST, and ALT/AST ratio were found with a 3.8-, 1.52-, and 3.08-fold increased risk for MetS compared with those with concentrations in the first tertile (OR = 3.80, 95% CI = 2.46 - 5.87 for ALT, OR = 1.52, 95% CI = 1.04 - 2.23 for AST, OR = 3.08, 95% CI = 2.05 - 4.63 for ALT/AST ratio). In subjects with elevated levels of GGT, the OR of MetS was 2.71 (95% CI = 1.80 - 4.09), which was significantly higher than that of the subjects in the first tertile. Similarly, in cases with elevated ALP levels, the OR of MetS was 1.64 (95% CI = 1.12 - 2.38). However, there was no significant association between the LDH level and MetS in the adjusted model.
| Liver Enzymes | Tertile 1 | Tertile 2 | Tertile 3 | P for Trend |
|---|---|---|---|---|
| ALT | ||||
| Range, U/L | (< 9.00) | (9.00 - 15.3) | (> 15.3) | |
| Crude | 1.00 | 1.69 (1.21 - 2.35) | 3.10 (2.24 - 4.28) | 0.001 |
| Adjusted modela | 1.00 | 1.65 (1.10 - 2.47) | 3.80 (2.46 - 5.87) | 0.001 |
| AST | ||||
| Range, U/L | (< 16.1) | (16.1 - 22.0) | (> 22.0) | |
| Crude | 1.00 | 1.32 (0.95 - 1.83) | 2.09 (1.53 - 2.85) | 0.001 |
| Adjusted model | 1.00 | 1.14 (0.77 - 1.69) | 1.52 (1.04 - 2.23) | 0.023 |
| ALT/AST | ||||
| Range | (< 0.50) | (0.50 - 0.81) | (> 0.81) | |
| Crude | 1.00 | 0.99 (0.72 - 1.37) | 2.04 (1.50 - 2.78) | 0.001 |
| Adjusted model | 1.00 | 1.13 (0.76 - 1.68) | 3.08 (2.05 - 4.63) | 0.001 |
| GGT | ||||
| Range, U/L | (< 14.0) | (14.0 - 21.9) | (> 21.9) | |
| Crude | 1.00 | 2.17 (1.54 - 3.06) | 4.61 (3.29 - 6.47) | 0.001 |
| Adjusted model | 1.00 | 1.56 (1.04 - 2.34) | 2.71 (1.80 - 4.09) | 0.001 |
| ALP | ||||
| Range, U/L | (< 155) | (155 - 203) | (> 203) | |
| Crude | 1.00 | 1.62 (1.18 - 2.23) | 2.71 (1.97 - 3.72) | 0.001 |
| adjusted model | 1.00 | 1.11 (0.76 - 1.62) | 1.64 (1.12 - 2.38) | 0.006 |
| LDH | ||||
| Range, U/L | (< 260) | (260 - 322) | (> 322) | |
| Crude | 1.00 | 1.08 (0.71 - 1.62) | 1.71 (1.15 - 2.56) | 0.007 |
| Adjusted model | 1.00 | 0.88 (0.52 - 1.47) | 0.98 (0.59 - 1.63) | 0.991 |
Abbreviations: ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate transaminase; GGT, gamma-glutamyl transpeptidase; LDH, lactate dehydrogenase.
aAdjusted for sex (male/female), age (years), BMI (kg/m2).
The results of the association between LFT and DM are presented in Table 3. After adjustment for confounding variables, subjects in the third tertile of ALT and ALT/AST ratio had a significantly increased risk of DM compared with subjects in the first tertile (OR = 4.32, 95% CI = 2.40 - 7.79 for ALT, OR = 3.28, 95% CI = 1.92 - 5.61 for ALT/AST ratio). In subjects with the highest levels of GGT in the third tertile, the risk of DM was significantly higher than the cases in the lowest tertile (OR = 2.52, 95% CI = 1.46 - 4.34). Elevated levels of ALP in the third tertile were also significantly associated with the higher risk of DM (OR = 1.74, 95% CI = 1.05 - 2.88). Serum concentrations of AST and LDH were not associated with DM in both crude and adjusted models.
| Liver Enzymes | Tertile 1 | Tertile 2 | Tertile 3 | P for Trend |
|---|---|---|---|---|
| ALT | ||||
| Range, U/L | (< 9.00) | (9.00 - 15.3) | (> 15.3) | |
| Crude | 1.00 | 1.24 (0.83 - 1.85) | 1.40 (0.94 - 2.11) | 0.089 |
| Adjusted modela | 1.00 | 1.79 (1.05 - 3.06) | 4.32 (2.40 - 7.79) | 0.001 |
| AST | ||||
| Range, U/L | (< 16.1) | (16.1 - 22.0) | (> 22.0) | |
| Crude | 1.00 | 0.80 (0.53 - 1.20) | 0.93 (0.63 - 1.37) | 0.812 |
| Adjusted model | 1.00 | 0.86 (0.51 - 1.45) | 0.94 (0.57 - 1.53) | 0.875 |
| ALT/AST | ||||
| Range | (< 0.50) | (0.50 - 0.81) | (> 0.81) | |
| Crude | 1.00 | 1.13 (0.76 - 1.68) | 1.30 (0.88 - 1.92) | 0.167 |
| Adjusted model | 1.00 | 1.60 (0.95 - 2.69) | 3.28 (1.92 - 5.61) | 0.001 |
| GGT | ||||
| Range, U/L | (< 14.0) | (14.0 - 21.9) | (> 21.9) | |
| Crude | 1.00 | 1.40 (0.90 - 2.18) | 2.69 (1.78 - 4.08) | 0.001 |
| Adjusted model | 1.00 | 1.12 (0.64 - 1.98) | 2.52 (1.46 - 4.34) | 0.001 |
| ALP | ||||
| Range, U/L | (< 155) | (155 - 203) | (> 203) | |
| Crude | 1.00 | 1.62 (1.03 - 2.54) | 3.50 (2.30 - 5.32) | 0.001 |
| Adjusted model | 1.00 | 0.90 (0.52 - 1.55) | 1.74 (1.05 - 2.88) | 0.021 |
| LDH | ||||
| Range, U/L | (< 260) | (260 - 322) | (> 322) | |
| Crude | 1.00 | 0.90 (0.53 - 1.54) | 1.14 (0.67 - 1.92) | 0.610 |
| Adjusted models | 1.00 | 0.54 (0.24 - 1.22) | 0.60 (0.27 - 1.33) | 0.243 |
Abbreviations: ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate transaminase; GGT, gamma-glutamyl transpeptidase; LDH, lactate dehydrogenase.
aAdjusted for sex (male/female), age (years), BMI (kg/m2).
The ORs (with 95% CI) of HTN and CKD across tertiles of LFT are shown in Tables 4 and 5, respectively. After adjustment for confounding variables, there were significant positive associations between risk of HTN and elevated levels of ALT (OR = 2.63, 95% CI = 1.66 - 4.17), GGT (OR = 2.01, 95% CI = 1.30 - 3.13), and ALP (OR = 1.90, 95% CI = 1.26 - 2.87). Also, higher LDH levels were associated with increased risk of CKD (OR = 2.43, 95% CI = 1.09 - 5.43). However, there was no significant association between HTN and CKD, and other liver enzymes.
| Liver Enzymes | T1 | T2 | T3 | P for Trend |
|---|---|---|---|---|
| ALT | ||||
| Range, U/L | (< 9.00) | (9.00 - 15.3) | (> 15.3) | |
| Crude | 1.00 | 1.23 (0.90 - 1.67) | 1.21 (0.89 - 1.65) | 0.207 |
| Adjusted modela | 1.00 | 1.46 (0.95 - 2.23) | 2.63 (1.66 - 4.17) | 0.001 |
| AST | ||||
| Range, U/L | (< 16.1) | (16.1 - 22.0) | (> 22.0) | |
| Crude | 1.00 | 1.10 (0.81 - 1.51) | 1.19 (0.88 - 1.62) | 0.226 |
| Adjusted model | 1.00 | 1.11 (0.73 - 1.70) | 1.32 (0.88 - 1.99) | 0.136 |
| ALT/AST | ||||
| Range | (< 0.50) | (0.50 - 0.81) | (> 0.81) | |
| Crude | 1.00 | 0.82 (0.60 - 1.12) | 0.82 (0.60 - 1.12) | 0.238 |
| Adjusted model | 1.00 | 0.84 (0.56 - 1.27) | 1.50 (0.99 - 2.29) | 0.058 |
| GGT | ||||
| Range, U/L | (< 14.0) | (14.0 - 21.9) | (> 21.9) | |
| Crude | 1.00 | 1.60 (1.15 - 2.21) | 2.23 (1.62 - 3.07) | 0.001 |
| Adjusted model | 1.00 | 1.33 (0.85 - 2.07) | 2.01 (1.30 - 3.13) | 0.001 |
| ALP | ||||
| Range, U/L | (< 155) | (155 - 203) | (> 203) | |
| Crude | 1.00 | 1.87 (1.33 - 2.62) | 3.29 (2.37 - 4.57) | 0.001 |
| Adjusted model | 1.00 | 1.21 (0.79 - 1.86) | 1.90 (1.26 - 2.87) | 0.001 |
| LDH | ||||
| Range, U/L | (< 260) | (260 - 322) | (> 322) | |
| Crude | 1.00 | 1.21 (0.81 - 1.81) | 1.79 (1.21 - 2.65) | 0.003 |
| Adjusted model | 1.00 | 0.84 (0.47 - 1.49) | 1.24 (0.73 - 2.11) | 0.358 |
Abbreviations: ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate transaminase; GGT, gamma-glutamyl transpeptidase; LDH, lactate dehydrogenase.
aAdjusted for sex (male/female), age (years), BMI (kg/m2).
| Liver Enzymes | T1 | T2 | T3 | P For Trend |
|---|---|---|---|---|
| ALT | ||||
| Range, U/L | (< 9.00) | (9.00 - 15.3) | (> 15.3) | |
| Crude | 1.00 | 0.81 (0.59 - 1.13) | 0.44 (0.30 - 0.64) | 0.001 |
| Adjusted modela | 1.00 | 0.96 (0.59 - 1.57) | 1.10 (0.64 - 1.92) | 0.711 |
| AST | ||||
| Range, U/L | (< 16.1) | (16.1 - 22.0) | (> 22.0) | |
| Crude | 1.00 | 1.15 (0.81 - 1.65) | 1.19 (0.84 - 1.69) | 0.275 |
| Adjusted model | 1.00 | 1.25 (0.73 - 2.12) | 1.43 (0.86 - 2.38) | 0.162 |
| ALT/AST | ||||
| Range | (< 0.50) | (0.50 - 0.81) | (> 0.81) | |
| Crude | 1.00 | 0.58 (0.41 - 0.80) | 0.35 (0.25 - 0.51) | 0.001 |
| Adjusted model | 1.00 | 0.73 (0.45 - 1.19) | 0.83 (0.48 - 1.42) | 0.383 |
| GGT | ||||
| Range, U/L | (< 14.0) | (14.0 - 21.9) | (> 21.9) | |
| Crude | 1.00 | 0.31 (0.92 - 1.87) | 1.31 (0.92 - 1.87) | 0.104 |
| Adjusted model | 1.00 | 0.92 (0.54 - 1.57) | 1.32 (0.77 - 2.25) | 0.195 |
| ALP | ||||
| Range, U/L | (< 155) | (155 - 203) | (> 203) | |
| Crude | 1.00 | 1.56 (1.08 - 2.27) | 2.11 (1.47 - 3.04) | 0.001 |
| Adjusted model | 1.00 | 0.81 (0.47 - 1.40) | 1.03 (0.62 - 1.72) | 0.675 |
| LDH | ||||
| Range, U/L | (< 260) | (260 - 322) | (> 322) | |
| Crude | 1.00 | 1.80 (1.06 - 3.03) | 2.50 (1.51 - 4.15) | 0.001 |
| Adjusted model | 1.00 | 1.87 (0.81 - 4.36) | 2.43 (1.09 - 5.43) | 0.033 |
Abbreviations: ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate transaminase; GGT, gamma-glutamyl transpeptidase; LDH, lactate dehydrogenase.
aAdjusted for sex (male/female), age (years), BMI (kg/m2).
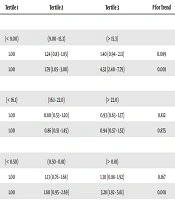
The association between levels of aminotransferases within their normal ranges and cardiometabolic disorders is presented in Table 6. In adjusted models, participants in the highest tertile of ALT had an increased risk of DM and HTN (OR = 3.99, 95% CI = 2.19 - 7.27 for diabetes, OR = 2.01, 95% CI = 1.31 - 3.10 for HTN), and participants in the highest tertile of AST had an increased risk of MetS (OR = 1.52, 95% CI = 1.04 - 2.23). The association between aminotransferases and CKD in crude or adjusted models was not statistically significant.
| Metabolic Syndrome | Diabetes | Hypertension | Chronic Kidney Disease | |||||
|---|---|---|---|---|---|---|---|---|
| Tertile 2 | Tertile 3 | Tertile 2 | Tertile 3 | Tertile 2 | Tertile 3 | Tertile 2 | Tertile 3 | |
| ALT | ||||||||
| Crude | 1.31 (0.94 - 1.83) | 1.82 (1.31 - 2.53)a | 1.27 (0.84 - 1.92) | 1.43 (0.94 - 2.16) | 1.36 (1.00 - 1.85)a | 1.38 (1.02 - 1.88)a | 0.76 (0.54 - 1.06) | 0.49 (0.34 - 0.71) |
| Adjusted Modelb | 1.03 (0.69 - 1.55) | 1.33 (0.89 - 2.00) | 1.97 (1.13 - 3.43)a | 3.99 (2.19 - 7.27)a | 1.56 (1.04 - 2.35)a | 2.01 (1.31 - 3.10)a | 0.87 (0.53 - 1.45) | 1.25 (0.72 - 2.16) |
| AST | ||||||||
| Crude | 1.32 (0.95 - 1.83) | 2.09 (1.53 - 2.85)a | 0.78 (0.52 - 1.18) | 0.85 (0.57 - 1.29) | 1.23 (0.90 - 1.67) | 1.38 (1.01 - 1.89)a | 1.31 (0.91 - 1.88) | 1.13 (0.78 - 1.65) |
| Adjusted Model | 1.14 (0.77 - 1.69) | 1.52 (1.04 - 2.23)a | 0.74 (0.43 - 1.27) | 0.87 (0.51 - 1.48) | 1.07 (0.71 - 1.61) | 1.29 (0.86 - 1.94) | 1.35 (0.78 - 2.34) | 1.22 (0.70 - 2.14) |
Abbreviations: ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate transaminase; GGT, gamma-glutamyl transpeptidase; LDH, lactate dehydrogenase.
aP value < 0.05.
bAdjusted for sex (male/female), age (years), BMI (kg/m2).
5. Discussion
In the present population-based study, we evaluated the odds of having cardiometabolic disorders, including MetS, DM, HTN, and CKD across tertiles of liver enzymes concentrations. Furthermore, the association between cardiometabolic disorders and aminotransferases within normal ranges of the enzymes were assessed. Our results showed that elevated serum concentrations of ALT, AST, ALT/AST ratio, GGT, and ALP were positively associated with an increased risk of MetS. Elevated serum concentrations of ALT, ALT/AST ratio, GGT, and ALP were also positively associated with the risk of DM. Also, higher levels of ALT, GGT, and ALP were positively associated with HTN. Moreover, there was a positive association between elevated levels of LDH and CKD.
These findings provided further evidence suggesting that elevated levels of liver enzymes may be associated with the risk of non-hepatic diseases, including MetS, DM, and HTN. The relationship between the risk of MetS and most of the liver enzymes has been reported in previous studies. In a cross-sectional study on Korean adults, elevated levels of ALT and AST were associated with MetS, and participants in the highest quartile of ALT and AST had a 7.90- and 3.81-fold increased prevalence of MetS, respectively (9). Also, in a large prospective cohort study in China, higher quartiles of GGT and ALT for both genders were associated with an increased risk of MetS (10). However, to the best of our knowledge, little is known about the association between the MetS and other liver enzymes, including ALP or LDH. In a cross-sectional study in Thailand, ALP level was associated with MetS, and subjects in the highest quartile of ALP had a 3.72-fold increased risk of MetS (29). The association between ALT/AST ratio and MetS or other cardiometabolic disorders has not been investigated in previous studies.
The association between LFT and DM has also been investigated. In a cohort study, elevated serum levels of ALT and GGT were associated with type 2 DM; ORs of diabetes were 1.49 and 1.58 in the highest quartile of ALT and GGT, respectively (12). The positive association between LFT, especially ALT and GGT, and DM, as well as the high frequency of elevated liver enzymes in diabetic patients, has also reported in several previous studies (14, 30-32).
The association between serum levels of liver enzymes, and HTN or CKD has been less documented. The ORs (95% CI) for elevated ALT and AST in subjects with HTN were 2.16 (1.93 - 2.65) and 1.68 (1.32 - 2.15), respectively (33). Also, a cohort study reported that participants in the highest quintile of GGT levels had a significantly higher risk for HTN (OR = 2.1; 95% CI = 1.1 - 4.0) (34). A case-control study to reveal the potential alterations in liver enzyme levels in CKD patients showed that ALT and AST levels were significantly lower, and ALP levels were significantly higher in CKD patients compared with healthy subjects (16). These results are not consistent with the results observed in our study.
Although the exact mechanism underlying the association between increased serum levels of liver enzymes and risk of MetS or its components remains unclear, the most probable explanation is the presence of non-alcoholic fatty liver disease (NAFLD) along with abnormal LFT, as well as the proven association between fatty liver and cardiometabolic disorders (35). Elevated liver enzymes are indicative of NAFLD, which is characterized by fat accumulation in the liver (36). Several studies have reported that NAFLD is associated with metabolic disorders, including DM, HTN, and dyslipidemia, which are defined as major components of MetS (37). On the other hand, increased free fatty acids concentrations in the liver can lead to dyslipidemia, as well as fasting hyperglycemia, insulin over-secretion from the pancreas, and a decrease in the efficiency of insulin signaling, which result in hyperinsulinemia and diabetes (3, 38, 39). Another possible mechanism to link LFT to cardiometabolic disorders is the liver inflammation following increased levels of liver enzymes (40), which can promote obesity and obesity-related disorders, such as MetS and DM by inflammatory pathways, including increased pro-inflammatory adipocytokines and decreased anti-inflammatory adiponectin (41). Notably, the cross-sectional design of the present study did not allow deriving any causal inferences, and cardiometabolic disorders possibly caused an elevation in the levels of liver enzymes.
In the present study, the observed association was stronger for elevated ALT than that of elevated AST and other liver enzymes, which can be explained by considerably longer plasma half-life of ALT or a higher specificity of ALT to liver disease (18, 42).
The association between aminotransferases (ALT and AST) levels within their normal ranges and cardiometabolic disorders were also investigated in the present study. Statistically significant associations were observed between elevated ALT levels and DM and HTN, as well as between elevated AST levels and MetS. The results of previous relevant studies are contradictory among different population groups and even between two genders (9, 19, 43). To the best of our knowledge, this is the first time to investigate the association between aminotransferases within normal ranges and the risk of DM, HTN, or CKD. The reference normal range of serum ALT concentration in our study was considered < 40 and < 34 U/L in men and women, respectively, and for AST it was considered < 34 U/L in both genders, according to a population-based study in Iran (28). It seems that cutoff levels of liver enzymes should be revised at lower levels and the modified values can help clinicians to use LFTs for early detection of non-liver-related disorders.
The current research was the first study to report the association between MetS, DM, HTN, CKD, and ALT, AST, ALT/AST ratio, GGT, ALP, and LDH, simultaneously. However, our study had some limitations. First, we conducted a cross-sectional study, which did not allow driving causal inferences. Second, other factors that could influence liver enzyme levels, such as taking herbal medicine or chemical drugs were not considered in our study. Third, the association between normal ranges of liver enzymes and cardiometabolic disorders was only assessed for ALT and AST due to the lack of valid reference normal ranges for other enzymes in the Iranian population. Future large-scale prospective studies are needed to reveal the association between elevated liver enzymes and cardiometabolic disorders.
5.1. Conclusions
In conclusion, we observed significant positive associations between elevated levels of ALT, AST, ALT/AST ratio, GGT, ALP, and MetS. Elevated serum concentrations of ALT, ALT/AST ratio, GGT, and ALP were also positively associated with the risk of DM. Also, elevated levels of ALT, GGT, and ALP were positively associated with HTN. Moreover, there was a positive association between elevated levels of LDH and the risk of CKD. Accordingly, based on the results of the present study, LFT can be helpful for the early detection of cardiometabolic disorders.