1. Background
The prevalence of HBV and HCV infections in people living with HIV (PLHIV) is higher than in the general population due to their common route of transmission. Although rates vary from one country to another, and even in different regions of the same country, it is reported that approximately 10% of PLHIV has HBV, and 20% - 30% has HCV co-infection in the world (1, 2).
HIV infection may elevate susceptibility to HBV and HCV infections, resulting in an increase in HBV and HCV viremia in case of co-infection; this may accelerate liver injury. HIV infection has a high negative effect on the progression of HBV-associated liver disease. Co-infected individuals are at a greater risk of mortality and morbidity than those who are mono-infected with HIV (3, 4). HIV/HBV co-infection is associated with reduced rates of spontaneous clearance, higher serum HBV DNA polymerase activity, lower rates of serum hepatitis B e antigen (HBeAg) loss, increased risk of cirrhosis due to low CD4 count, risk of hepatocellular carcinoma, and hepatic mortality (5, 6).
Spontaneous clearance of HCV is less common in HCV co-infected patients who do not receive antiretroviral therapy (ART). These patients have higher HCV viral load and progress faster than those without HIV infection. Although ART reduces HCV-associated mortality in co-infected patients, the presence of HCV infection may complicate ART and elevate the risk of drug-related hepatotoxicity (7).
Turkey is one of the countries with a low HIV prevalence, despite the significant increase in the incidence of HIV infection in recent years. From 1985, when the first case was detected, to 2018, a total of 19,748 PLHIV were reported, and the number of new HIV positive cases in 2015 and 2018 were 2048 and 3356, respectively (8).
Thanks to effective vaccination, screening, and awareness programs conducted throughout the past 20 years, Turkey has transformed from a high endemic country to an intermediate endemic country concerning HBV prevalence, while the prevalence of HCV infection is less than 1% (9). Although Turkey is a major immigrant-receiving country due to its geographical location, a significant proportion of immigrants also use the country as a route to Western countries. Istanbul, where our hospital is located, is the most populous city receiving migration from every region of the country with a population of more than 15 million people. The population of patients that we are following reflects Turkey’s general population.
2. Objectives
This study aimed to examine the prevalence of HCV and HBV co-infection and its risk factors, as well as characteristics of a population of PLHIV, in the context of rapidly changing sociodemographic factors and limited literature data.
3. Methods
This study was undertaken at a centrally located general research and training hospital in Istanbul. Istanbul is a city with approximately 20% of Turkey’s population, which receives migration from all over the country. No hospital in Turkey only serves HIV patients. There are a total of 13 centers in Istanbul serving HIV-positive patients, including our center. A total of 717 treatment-naïve patients who were followed up at our outpatient unit between January 2015 and July 2018 were included in the study. Information on age, risk factors for HIV transmission such as sexual and intravenous routes, education level, routine laboratory tests such as CD4 lymphocyte count, HIV viral load, and hepatitis B and C serological markers were retrospectively collected from the medical records of the patients. Laboratory investigations were performed at our central laboratory using the same test kits in all the patients. Cases with HBsAg positivity were identified as having HBV co-infection, and those with HCV RNA in anti-HCV positive cases were identified as having HCV co-infection. All the patients with anti-HBc positivity were considered to be exposed to HBV. Based on the European Consensus guidelines, patients with CD4 counts < 350 cells/mm3 were regarded as late presenters (10). To evaluate the participants’ education level, primary school was defined as being graduated only from a school that includes ≤ 8 years.
The CD4+ T lymphocyte count was determined using standard flow cytometric methods (Beckman Coulter Inc., Fullerton, California, ABD), while HIV RNA, HBV DNA, and HCV RNA were determined with the polymerase chain reaction methodology (Artus HIV virus-1 QS-RGQ Kit, Qiagen, Germany; Artus HBV QS-RGQ Kit, Qiagen, Germany; and Artus HCV QS-RGQ Kit, Qiagen, Germany, respectively).
3.1. Statistical Analyses
Discrete variables were expressed as frequency and percentage, while continuous variables were expressed as arithmetic mean and standard deviation. The discrete variables were evaluated with chi-square and Fisher’s exact test. Normal distribution of the continuous variables was tested with the Kolmogorov-Smirnov test, and those without a normal distribution were assessed with the Mann-Whitney U test. A P value of less than 0.05 was considered statistically significant. A univariate analysis was performed to analyze differences between HIV mono-infection and HIV/HBV co-infection groups in terms of age, gender, risk factors for HIV transmission, CD4 count, education status, and HIV RNA level. The statistical package for social sciences (IBM SPSS Corp.; Armonk, NY, ABD) v. 23.0 was used for statistical evaluations.
4. Results
Of the 717 patients followed up with a diagnosis of HIV/AIDS, 645 (89.9%) were male. The mean age patients was 35.94 ± 11.69 years, and 377 patients (52.5) were men who had sex with men (MSM). The mean age was 35.9 ± 11.68 years in the MSM patients and 40.58 ± 11.67 years in the HS patients. There were no people who injected drugs (PWID). The median HIV RNA level of the patients was 150.076 (IQR:38226-620.520) copy/ml, and there were 270 (37.6%) late presenters. No risk factors for HIV infection could be detected in 29 subjects (4%). Of the patients, four (0.5%) had HCV and 32 (4.5%) had HBV co-infection. Moreover, triple co-infection with HIV, HBV, and HCV was not detected in any of the patients, and also none of the patients co-infected with HBV had HDV infection. Among the 717 patients, there were 57 patients whose anti-HBc IgG or anti-HBs results could not be achieved. Therefore, the HBV serologic status of only 660 of the patients is shown in Table 1.
| Hepatitis B Serology | Values |
|---|---|
| HBsAg+, anti-HBc+, anti-HBs- | 32 (4.8) |
| HBsAg-, anti-HBc+, anti-HBs+ | 126 (19) |
| HBsAg-, anti-HBc+, anti-HBs- | 59 (8.9) |
| HBsAg-, anti-HBc-, anti-HBs+ | 167 (25.3) |
| HBsAg-, anti-HBc-, anti-HBs- | 276 (41.8) |
| Total | 660 (100) |
aValues are expressed as No. (%).
Table 2 shows a comparison of patients infected with HIV alone or co-infected with HIV and HBV in terms of risk factors. As can be observed from the table, there were statistically more heterosexual patients among the co-infected group (P = 0.03, OR = 2.23, %95; 95% CI = 1.07 - 4.65). No significant associations could be observed between age, gender, educational status, HIV RNA level, late presentation, and co-infection (P = 0.07, P = 0.06, P = 0.64, P = 0.22, and P = 0.25).
| HIV Mono Infection (N = 685) | HIV/HBV Co-Infection (N = 32) | Odds Ratio | 95% CI | P | |
|---|---|---|---|---|---|
| Age, y | 33 (28 - 42) | 37 (29 - 46) | 0.07 | ||
| Gender | 1.05 | 1.03 - 1.07 | 0.06 | ||
| Male | 613 (89.4) | 32 (100) | |||
| Female | 72 (10.6) | 0 (0) | |||
| Education | 0.64 | ||||
| Primary school | 182 (36.0) | 11 (44.0) | |||
| Secondary school | 114 (22.6) | 4 (16.0) | |||
| University | 209 (41.4) | 20 (62.5) | |||
| Heterosexual transmission | 271 (42.7) | 20 (62.5) | 2.23 | 1.07 - 4.65 | 0.03b |
| HIV RNA, copies/mL | 0.22 | ||||
| (38214 - 599478) | 146730 | ||||
| (16787 - 821356) | 201616 | ||||
| CD4 T lymphocyte < 350 cells/mm3 | 245 (39.4) | 15 (50) | 1.53 | 0.73 - 3.20 | 0.25 |
Abbreviation: IQR, inter quartile range.
aValues are expressed as median (IQR) or No. (%).
bSignificant.
Table 3 shows a comparison of the MSM and HS patients in terms of age and late presentation. The HS patients were significantly older and more likely to be late-presenters (P < 0.001) compared to their MSM counterparts.
| MSM (N = 377) | HS (N = 311) | P | |
|---|---|---|---|
| Age, y | 32.5 ± 9.6 | 40 ± 11.8 | < 0.001 |
| CD4 T lymphocyte < 350 cells/mm3 | 130 (33.8) | 140 (47.9) | < 0.001 |
aValues are expressed as mean ± SD.
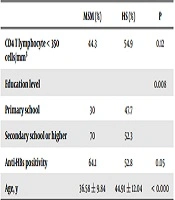
Table 4 shows a comparison of the HBV-exposed patients in terms of risk factors for HIV transmission. In 217 of the PLHIV (30.2%), anti-HBc was positive, and these individuals were considered to be previously exposed to HBV. MSM patients exposed to HBV had a significantly higher level of education (P = 0.008). Moreover, the rate of resolved infection was markedly higher in the MSM subjects, as compared to the HS subjects (P = 0.05).
| MSM (%) | HS (%) | P | |
|---|---|---|---|
| CD4 T lymphocyte < 350 cells/mm3 | 44.3 | 54.9 | 0.12 |
| Education level | 0.008 | ||
| Primary school | 30 | 47.7 | |
| Secondary school or higher | 70 | 52.3 | |
| Anti-HBs positivity | 64.1 | 52.8 | 0.05 |
| Age, y | 36.58 ± 9.84 | 44.91 ± 12.04 | < 0.000 |
aValues are expressed as mean ± SD.
5. Discussion
Currently, there are around 36.7 million HIV-infected people worldwide, with 1.8 million people suffering this disease each year and 1 million subjects dying from AIDS (11). Based on the data provided by the Turkish Ministry of Health, there have been approximately 21,520 HIV/AIDS cases as of 31 December 2018. Although there has been a significant increase in the number of new HIV infected cases in Turkey since 2014, it remains a low-prevalence country (8). It is known that around 240 million people worldwide have chronic HBV infection and 130 to 170 million people have chronic HCV infections, according to the WHO data (12). In epidemiological studies examining the seroprevalence of HBsAg, a prevalence ranging from 2% to 7% has been reported, varying between regions in Turkey. In general, the prevalence of HBV in the eastern part of Turkey is higher than 4% (13). In the present study, 4.5% of the PLHIV had HBV co-infection, consistent with the figures reported in the general Turkish population.
Approximately 37 million people are infected with HIV globally, and 5% - 20% of them are also co-infected with HBV. Rates of chronic HBV in HIV-infected individuals vary significantly between regions and risk-based groups, reflecting different patterns of transmission. In regions with a high prevalence of HBV such as Sub-Saharan Africa and East Asia, most HBV infections are acquired through the perinatal route or via cultural means such as close household contact, scarification, or tattooing. In these patients, HBV infection is more likely to progress into chronic infections, resulting in a high prevalence of chronic HBV infection in a young population at risk of sexually transmitted HIV (14). In low HBV prevalence areas such as North America, Western Europe, and Australia, HBV infection is mainly acquired in adulthood in high-risk groups, i.e., PWID with multiple HS partners and MSM. Chronic HBV infection occurs in 6% to 14% of PLHIV (6, 15). In a study from the United States involving 15 states with low HBV prevalence and 504,398 PLHIV, 2% of the subjects were found to have HBV co-infection, which was more common in males, MSM subjects, and those between 40 and 49 years of age (2). In an Italian study with 1402 PLHIV, the prevalence of HBV co-infection was reported to be 4.1%, similar to our observations. It appears from these studies that the major risk factor for HIV/HBV co-infection is sexual exposure (1). In a Romanian cohort with similar endemics data, i.e., low HIV and intermediate HBV prevalence, the HIV/HBV co-infection rate was 19%, which is much higher than in our study. The high PWID rate (15%) in the Romanian cohort, which is different from our study, may explain this difference (16). In another study from Nepal involving 677 PLHIV, the HBV seroprevalence was 4.4%, similar to our observations. In that study, the rate of HBV co-infection was significantly higher in males and PWID (17). When the databases were systematically reviewed between 1996 and 2012 in Iran, one of the border neighbors of Turkey, the highest prevalence of HIV/HBV (1.88%), was among PWID (18). In a cohort study in Greece involving 737 HIV-positive patients, the percentage of HBsAg-seropositivity was 12.1%. In that study from another neighboring country with a similar number of patients, the HBV co-infection rate was higher than in our study, and the majority of the cases were MSM subjects (19). The rate of HBV co-infection detected in our study was lower when compared with countries with similar HBV endemicity, and PWID and MSM were not risk factors associated with HBV co-infection. In a study by Karaosmanoglu et al. (20) with 209 HIV-infected individuals from Turkey, the rate of HBV co-infected patients was 4.3%, which is similar to our study. In that study, 33.9% of the patients had HBV exposure, which was again similar to our figures (30.12%). In addition, in their study, no statistically significant difference was observed in the prevalence of HBV in patients with late presentation compared to those in early presentation (20).
In our study, the prevalence of HBV co-infection was significantly higher in the HS subjects compared to other studies, which may be related to delayed diagnosis and advanced age of HS subjects. We believe that a statistically higher level of education in our MSM patients contributed to the use of screening tests and early diagnosis of the patients already at risk for HIV infection. Although HBV exposure was similar between the MSM and HS patients, the latter group had a higher frequency of HBV infection, suggesting that the infection is more likely to have a chronic course in those with a delayed diagnosis (5, 6).
In our patient population, late presentation was not associated with an increased risk for HIV/HBV co-infection. After examining patients with previous exposure to HBV, no difference was revealed between the HS and MSM subjects. The statistical difference in the HS patients in terms of HBV co-infection can be explained by the significant late presentation of the HS patients among all the patients. This supports the fact that HBV infection cannot be effectively controlled in immunocompromised patients, and the risk of HBV reactivation is high in these patients.
Globally, there are approximately 2,278,400 patients with HIV/HCV co-infection, while the prevalence of HCV infection in the general population is reported to be 2.4%. Among patients with HCV co-infection, 82.4% are PWID, and the probability of HCV infection is six-fold higher in PLHIV than in HIV-negative individuals (7). Turkey is among the countries with low endemicity in terms of both HIV and HCV, which is reported to have an HCV prevalence of approximately 1% (9). In a study from Turkey with HIV-infected individuals, the prevalence of HCV co-infection was similar to that in the general population (0.9%) (21). In our study, the prevalence of HIV/HCV co-infection was found to be similar to this figure. The low prevalence of HCV co-infection in our patients may be related to the absence of PWID, which is a high risk for HCV transmission. Since there were only a few patients with HCV co-infection in our study, factors associated with HCV infection were reported but not included in the statistical analyses, which may be considered as a limitation of our study.
5.1. Conclusions
In our study, the prevalence of HBV co-infection among HIV infected patients was similar to that in the general population, and the rate of HBV seropositivity was higher among those with a heterosexual route of transmission. A high occurrence of HBV co-infection in HS patients with late presentation suggests that HBV infection is more likely to have a chronic course in individuals with a delayed diagnosis of HIV/AIDS. The high educational level of our MSM patients at risk for HIV/AIDS enabled them to be diagnosed early and vaccinated against HBV. In the past 5-year period, a significant increase has been observed in the incidence of HIV infection, and in Turkey with intermediate endemicity for HBV, education, early diagnosis and protection are essential for the control of these two infections that share common routes of transmission. Therefore, awareness-raising and early diagnosis in the general population, especially in risk groups, should represent a significant step in the control of these diseases with severe consequences.