1. Context
Hepatitis C virus (HCV) is one of the most severe threats to global health outcomes, affecting approximately 71 million patients worldwide in 2015 (1). HCV, if left untreated, patients living with the condition may progress to end-stage liver diseases, such as cirrhosis and hepatocellular carcinoma. Importantly, genotype diversity directly affects the clinical course of infected patients. For instance, HCV-genotype 3 (GT3) is associated with a relatively aggressive clinical course of liver disease, with a high risk of fibrosis progression, severe steatosis, and/or hepatocellular carcinoma (2, 3). There are seven genotypes and 34 subtypes of HCV identified to date (4, 5), the most prevalent genotypes (GT) in the world are GT1 and GT3, with GT3 accounting for 17.9% of HCV infected patients.
In recent years, remarkable progress has been made in identifying treatment options for HCV, particularly given the development of direct-acting antivirals (DAA) treatments. The World Health Organization (WHO) has set a goal for HCV to be eliminated by 2030. However, HCV-GT3 appeared to be more difficult to cure HCV, compared to the other HCV genotypes in the earlier era of DAA, which required a longer duration of treatment and the addition of ribavirin (6). Thus, GT3 acts as a barrier to curb this pandemic disease worldwide. The sofosbuvir/velpatasvir (SOF/VEL) combination, is the first regimen to be approved for the treatment of pan-genotype HCV infection. Sofosbuvir (SOF; Sovaldi®, Gilead, Forster City, CA) is a nonstructural protein 5B (NS5B) inhibitor, and velpatasvir (VEL; Gilead) is a nonstructural protein 5A (NS5A) inhibitor. This combination is given as a single tablet regimen, known as Epclusa® (Gilead), which acts as a pan-genotypic inhibitor with potent in vitro activity against the HCV-GT3. Compared to earlier standard regimens for HCV, the effectiveness of this single-tablet increases for HCV-GT3 patients. (e.g., 24 weeks of SOF plus ribavirin resulted in a sustained virological response (SVR) rate of 81% in the ASTRAL-3 clinical trial and 12 weeks of sofosbuvir plus daclatasvir resulted in an SVR (i.e., SVR12) rate of 89% in the ALLY-3 clinical trial) (7). The regimen of SOF/VEL has other advantages, one being the fact that it is an oral, once-daily interferon-free treatment, well-tolerable, and of short duration. SOF/VEL is now the standard of care (SoC) regimen for patients with HCV-GT3 recommended by both the European Association for the Study of the Liver (EASL) (8) and the American Association for the study of liver diseases (AASLD) (9).
However, patients with HCV-GT3 infection, particularly those with high risk of lower SVR (e.g., presenting cirrhosis of the liver, with a previous history of anti-HCV treatment, and/or with a baseline resistance-associated variant substitutions [RAVs]), have emerged as a difficult-to-cure population (10). Notably, the current recommendations for SOF/VEL treatment for the HCV-GT3 subgroup of patients are inconsistent. For instance, treatment-naïve patients with HCV-GT3 and compensated cirrhosis were recommended a daily fixed-dose combination of SOF/VEL lasting 12 weeks by AASLD (9), but not by EASL (8). Thus, our analysis aimed to systematically examine the current data, in order to assess the effectiveness of SOF/VEL in HCV-GT3 infected patients.
2. Objectives
Here, we evaluated the SVR rates obtained from the fixed-dose combination of SOF/VEL in HCV-GT3 patients and provided evidence for clinical guidelines.
3. Data Sources
3.1. Search Strategy
Using the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines, we designed a comprehensive search strategy to identify relevant published studies (11). For this, we searched PubMed/MEDLINE, Embase, and the Cochrane Library for studies from inception until May 3rd, 2019. There were no restrictions on language or publication date. In PubMed, we combined free text words and medical subject headings (MESH) describing the study population and the intervention. The search strategy in PubMed is listed in Box 1. This same strategy for our search was adapted for the Cochrane Library, as well as for EMBASE searches. Grey kinds of literature were manually searched for annual conferences of the EASL, AASLD, the Asian Pacific Association for the Study of the Liver (APASL), and WHO, which were restricted to one year, from April 2018 to May 2019. Finally, we checked the reference lists of relevant articles manually for potential eligible studies that could contribute to our research.
| Pubmed Search Keywords | Query |
|---|---|
| “sofosbuvir” and “velpatasvir” and“hepatitis C” and “HCV” AND “Genotype 3” and “GT 3” | (((“genotype”[MeSH Terms] OR “genotype”[All Fields]) AND 3[All Fields]) OR “gt 3”[All Fields]) AND (((((((“hepatitis c”[MeSH Terms] OR “hepacivirus”[MeSH Terms]) OR (“hepatitis c”[MeSH Terms] OR “hepatitis c”[All Fields] OR “hepacivirus”[MeSH Terms] OR “hepacivirus”[All Fields])) OR “hepacivirus”[MeSH Terms]) OR (“hepacivirus”[MeSH Terms] OR “hepacivirus”[All Fields])) OR HCV[All Fields]) AND (“velpatasvir”[Supplementary Concept] OR (“velpatasvir”[Supplementary Concept] OR “velpatasvir”[All Fields]))) AND ((“sofosbuvir”[MeSH Terms] OR “sofosbuvir”[All Fields]) OR “sofosbuvir”[MeSH Terms])) |
4. Study Selection
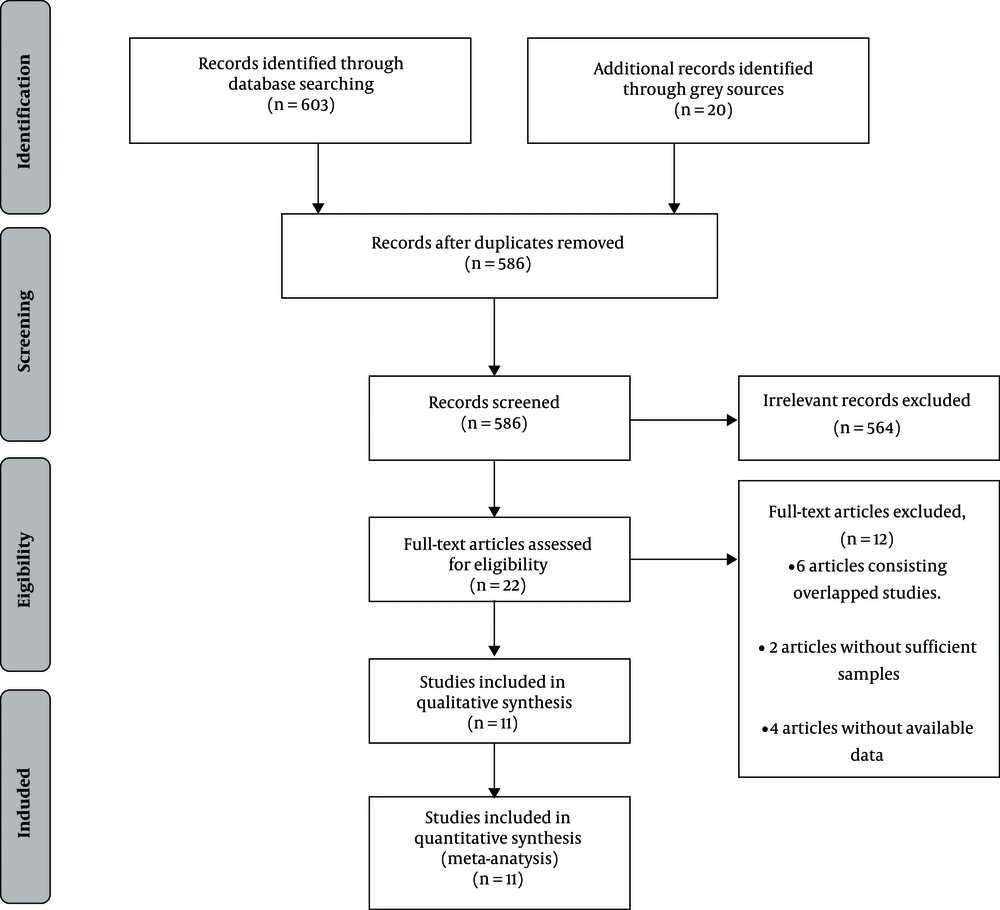
Bibliographic data and abstracts of all records found were downloaded into MedRef V5. Duplicated citations were excluded after software screening. The title and abstract of every record were screened independently by two reviewers (LW and R). After obtaining the full text of the manuscript identified in the initial screen, the same reviewers assessed the eligibility independently by reviewing the full text. Discrepancies between reviewers were dealt buy reaching a consensus. Studies consisting of a mixture of genotypes, which did not report HCV-GT3 specific data were excluded. In addition, we identified overlapping reports by matching study names, trail numbers, authors, among other factors, then chose reports with complete data and analyses. Other including and excluding criteria are given in detail in Table 1. Of note, if the sample size of the study was less than 30, we also excluded it from our analysis (12-14).
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Adult patients(definition by studies) chronically with HCV GT3 | Patients with acute HCV infection |
| The numbers of samples are more than 30. | In vitro study | |
| Non-GT3 infection or lack of GT3-specific stratification | ||
| Intervention | A fixed-dose combination tablet containing sofosbuvir (400 mg/d) and velpatasvir (100 mg/d) for 12 weeks | A regimen consisting of ribavirin or Voxilaprevir |
| Treatment duration is not 12 weeks | ||
| Outcome | SVR12a or mITTb or PPc | If the primary outcomes were not mentioned |
| Type of study | RCT or cohort or real-world study | Pharmacokinetics studies |
| Cost-effectiveness studies | ||
| Case-control reports | ||
| Reviews, editorial | ||
| Studies without full-text |
aHCV RNA level is below the lower limit of quantification at 12 weeks after the end of treatment.
bModified intention-to-treat.
cPer-protocol analysis.
5. Data Extraction
Data to be extracted included the name of the first author, year of publication, study design, country, number of patients, age, sex, HCV RNA levels, drug dosage, duration of treatment in each group, HCV genotype, demographic characteristics, HIV co-infection, the presence of cirrhosis, previous treatments, baseline NS5A resistance-associated variants, overall SVR rates, and SVR sub-analysis. One reviewer (LW) extracted data into Microsoft Excel tables that were checked by the other reviewer (RX).
5.1. Assessment of the Bias Risk of Included Studies
Here, we used the Critical Appraisal Skills programme (CASP) to assess the included literature for quality assessment (15). The CASP checklist was based on 12 aspects obtained from 14 questions, as follows: (1) Did the study address a clearly focused issue?; (2) was the cohort recruited acceptably?; (3) was the exposure accurately measured to minimize bias?; (4) was the outcome accurately measured to minimize bias?; (5A) have the authors identified all the important confounding factors?; (5B) have the authors taken into account the confounding factors in the design and/or analysis?; (6A) was the follow up of subjects complete enough?; (6B) was the follow up of subjects long enough?; (7) what were the results of this study?; (8) how precise were the results?; (9) do you believe the results?; (10) can the results be applied to the local population?; (11) do the results of this study fit with the other available evidence?; (12) what are the implications of this study for clinical practice?
In addition, every question had three options, which were, yes, no, or unclear. Discrepancies regarding the CASP checklist were resolved by consultation with an additional reviewer (XHY) (16).
5.2. Statistical Analysis and Bias Risk of Individual Studies
This meta-analysis was carried out using the R software version 3.6.0. To make sure the rate estimate was consistent with the normal distribution, we performed a normality text and selected the best transformation to adapt it, then synthesized the rate with a 95% confidence interval (CI). Heterogeneities between trials were assessed using the Q test (17). If I2 was greater or equal to (≥) 50% and the P value was less than (<) 0.05, the data was defined as a statistically significant degree of heterogeneity. The data were then pooled by using a random-effects model and sensitivity analyses were performed. Finally, the publication bias was assessed using the funnel plot and Egger tests. A P value of < 0.05 was considered statistically significant.
6. Results
After our initial search, 623 records were identified, of which 37 citations were excluded for duplication, 535 records were eliminated for irrelevancy, and 29 conference abstracts were excluded for lack of full-text. Other details of this procedure are shown in a flow diagram of study identification presented in Figure 1. Finally, 11 studies, reported in 10 publications met the inclusion and exclusion criteria and were included in our analysis (10, 18-26).
6.1. Characteristics of Studies and Patients
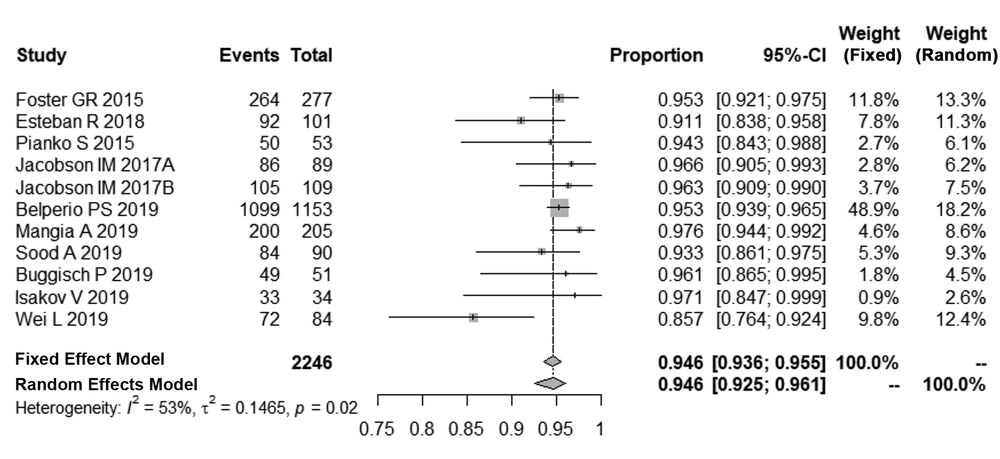
In total, 11 studies were assessed in this meta-analysis, including five randomized controlled trials (RCTs), one real-world cohort, one single-center cohort, and four multi-center cohorts. Ten out of the 11 studies selected were funded by Gilead Sciences, which manufactures the combination of SOF/VEL, with one real-world cohort (21). In total, a patient population of 2246 was investigated in the studies. The characteristics of the studies and patients enrolled are listed in Table 2. The range of clinical sites included Europe, North America, Asia, and Australia. Nine out of eleven (9/11) trials recruited patients presenting compensated cirrhosis and the GT3 (10, 18-22, 24-26). In addition, two studies included a small number of patients with Child-Pugh B cirrhosis (21, 22); 10 studies recruited patients with GT3, who had previous anti-HCV treatment, with most of the patients having a history of previous treatments with interferon-based regimens (10, 18-22, 24-26). Notably, seven trials included patients with GT3 and baseline NS5A RAVs (10, 18-20, 24, 26), although the proportions of these subgroups varied and are listed in detail in Table 3.
| Study | Location | Study Design | Number of Patients | Compensated Cirrhosis, % | Previous HCV Treatment, % | Baseline NS5A RAVs, % |
|---|---|---|---|---|---|---|
| Foster (18) | Countriesa | RCT | 277 | 28.9 | 25.6 | 15.5 |
| Esteban (10) | Spain | RCT | 101 | 100 | 26.7 | 18.8 |
| Pianko (20) | Countriesb | RCT | 53 | 49.1 | 100 | 0 |
| Jacobson (A) (19) | Countriesc | RCT | 89 | 0 | NA | NA |
| Jacobson (B) (19) | Countriesc | RCT | 109 | 100 | 29.4 | 17.4 |
| Belperio (21) | USA | Real-world cohort | 1153 | NA | NA | NA |
| Mangia (22) | Italy | Multi-center cohort | 205 | 100 | 26.8 | NA |
| Sood (23) | India | Multi-center cohort | 90 | NA | NA | NA |
| Buggisch (24) | German | Single-center cohort | 51 | NA | NA | NA |
| Isakov (25) | Russia, Sweden | Multi-center cohort | 34 | 32.4 | NA | NA |
| Wei (26) | Asian countriesd | Multi-center cohort | 84 | NA | NA | 54.8 |
aUSA, Canada, France, Germany, Italy, The United Kingdom, Australia, and New Zealand.
bUSA, Australia, and New Zealand.
cUSA, Canada, France, Germany, The United Kingdom, Australia, and New Zealand.
dChina, Thailand, Vietnam, Singapore, and Malaysia.
Importantly, most of the included studies were of high-quality, however, three studies did not identify all critical confounding factors due to the lack of data on NS5A RAVs at baseline (21-23). The results obtained from the last question were unclear for observational studies, given that one observational cohort hardly provided sufficiently strong evidence to recommend clinical practice changes. In addition, one trial conducted in Asia had an obvious limitation (26), the subpopulation with cirrhosis and HCV subtype 3b were over-represented, based on the enrolment design, because of this, the data could not be applied to the local population. It was also unclear whether the results of this trial could fit with the other available evidence. Results obtained from the CASP checklist for included studies are listed in Table 3.
| Study | 1 | 2 | 3 | 4 | 5a | 5b | 6a | 6b | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foster (18) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Esteban (10) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Pianko (20) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Jacobson (A) (19) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Jacobson (B) (19) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Belperio (21) | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Unclear |
| Mangia (22) | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Unclear |
| Sood (23) | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Unclear |
| Buggisch (24) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Unclear |
| Isakov (25) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Unclear |
| Wei (26) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Unclear | Unclear |
Abbreviations: N, no; Y, yes.
6.2. Outcomes
6.2.1. Primary Outcomes
Sustained virological responses (SVRs): The dominant outcome measure used was the rate of the SVR12 post-treatment. However, if the data on modified intention-to-treat (mITT) and per-protocol analysis were available, the data on mITT was used first. Importantly, all the SVR12 rates of the included studies were calculated by mITT.
A test of normality indicated that the best transformation was logit, which was consistent with a normal distribution. After logit transformation, the pooled SVR12 rates in patients with HCV-GT3 was 94.6% with a random effect model by inverse method (95% Cl, 92.5% - 96.1%, I2 = 53%, P = 0.02). The details of these analyses are shown in Figure 2.
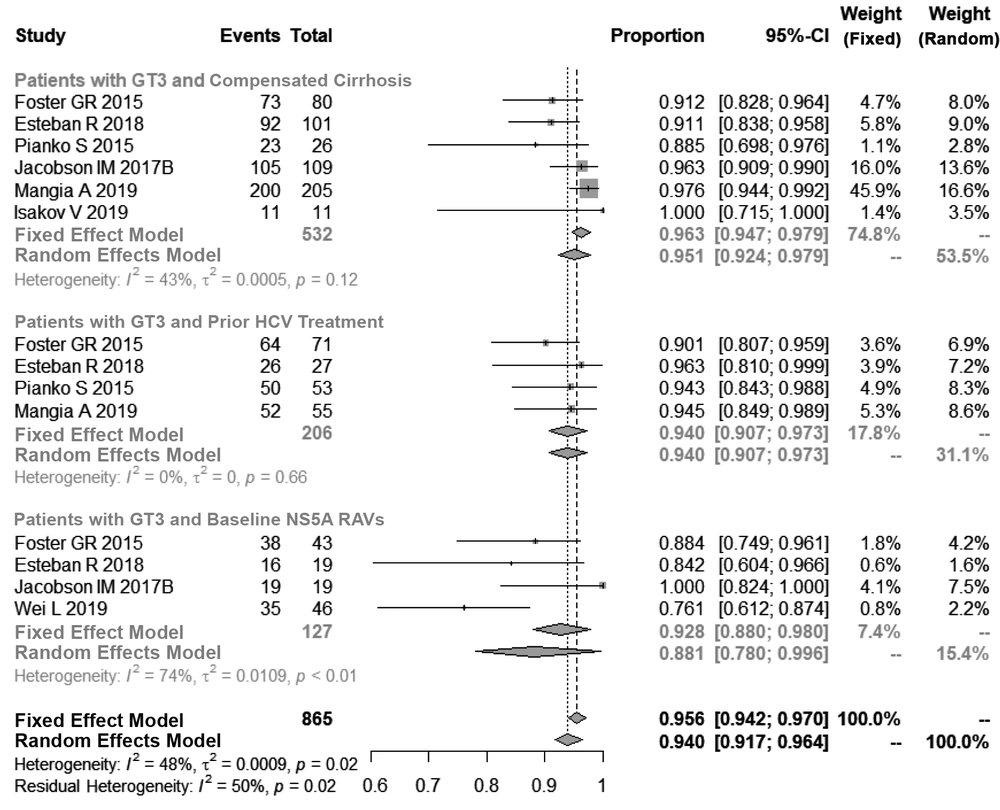
Subsequently, we performed a subpopulation analysis. The pooled SVR12 rate in patients with compensated cirrhosis and HCV-GT3 was found to be 96.3% (95% Cl, 93.0% - 96.3%, I2 = 43%, P = 0.12). Similarly, for patients with HCV-GT3, who had obtained prior anti-HCV treatment, the SVR12 rate was achieved in 94.0% of patients (95% Cl, 90.7% - 97.3%, I2 = 0%, P = 0.66). For the HCV-GT3 subpopulation and baseline NS5A RAVs, the SVR12 rate was found to be 88.1% (95% Cl, 78.0% - 99.6%, I2 = 74%, P < 0.01) as seen in Figure 3.
6.3. Sensitivity Analysis and Publication Bias
There were significant heterogeneities between the 11 studies (I2 = 53%, P = 0.02), which were most significant in the data presented in Figure 2. Therefore, we performed an influential analysis, the detailed the results in Table 4. Here, the data indicated that the SVR12 rate was increased to 95.2% and I2 was reduced to 0% after omitting one study by Wei et al. (26), indicating that this study was the biggest source of heterogeneities in our meta-analysis.
| Proportion | 95% CI | tau2 | I2 | |
|---|---|---|---|---|
| Omitting Foster (18) | 0.945 | [0.920; 0.962] | 0.193 | 0.569 |
| Omitting Esteban (10) | 0.949 | [0.928; 0.964] | 0.143 | 0.513 |
| Omitting Pianko (20) | 0.946 | [0.924; 0.962] | 0.167 | 0.574 |
| Omitting Jacobson (A) (19) | 0.944 | [0.922; 0.960] | 0.158 | 0.560 |
| Omitting Jacobson (B) (19) | 0.944 | [0.921; 0.960] | 0.162 | 0.562 |
| Omitting Belperio (21) | 0.945 | [0.919; 0.963] | 0.221 | 0.530 |
| Omitting Mangia (22) | 0.941 | [0.919; 0.958] | 0.125 | 0.493 |
| Omitting Sood (23) | 0.947 | [0.925; 0.963] | 0.171 | 0.568 |
| Omitting Buggisch (24) | 0.945 | [0.923; 0.961] | 0.162 | 0.570 |
| Omitting Isakov (25) | 0.945 | [0.923; 0.961] | 0.157 | 0.567 |
| Omitting Wei (26) | 0.952 | [0.942; 0.960] | 0.000 | 0.000 |
| Pooled estimate | 0.946 | [0.925; 0.961] | 0.146 | 0.527 |
Furthermore, we used the funnel plot and Egger test to test the publication bias. The details of the funnel plot are shown in Figure 4. The P value of the Egger test for funnel plots asymmetry was not found to be statistically significant (t = 0.10398, df = 9, P value = 0.9195).
7. Conclusions
It has been reported that the HCV-GT3 is a highly common infection worldwide, which accounts for 22.0% of HCV patients. Interestingly, the world distribution of HCV-GT3 is unequal, with the highest prevalence found in South Asia, 79.0% in Pakistan and 54.4% in India (27). These data indicate that a substantial number of patients are affected by HCV-GT3, and the majority of them live in low-income countries. HCV-GT3 is commonly transmitted among people who inject recreational drugs and share needles, which are a curial reservoir for the infection (28). These epidemiological characteristics make HCV-GT3 harder to treat and control.
Our meta-analysis demonstrates the high effectiveness of the SOF/VEL regimen in patients with HCV-GT3 and supports the current guidelines put forth by EASL and AASLD, both of which recommend the combination of SOF/VEL as the SoC for treatment-naïve patients with HCV-GT3. Compared to previous treatment options, this single-tablet regimen (STR) significantly improves the SVR12 rate of HCV-GT3 patients. Furthermore, an oral pan-genotypic treatment of short duration and with good tolerability could remove the need for upfront genotype testing, thus reducing monitoring costs, improving therapeutic adherence and allowing more patients to become eligible for treatment. It may also facilitate the elimination of HCV infection in low-resource regions, in which GT3 has been shown to have a high prevalence.
Notably, only one study (26) in our meta-analysis enrolled the majority of patients with subtype 3b. However, the remaining studies rarely consisted of these patients. Our sensitivity analysis suggests that the study (26) conducted in Asia significantly reduced the pooled SVR12 rate. In this study, the SVR12 rate was found to be 85.7% (72 out of 84) in patients with HCV-GT3, and 76.2% (32 out of 42) in patients with subtype 3b. In addition, 50% (7 out of 14) of cirrhotic patients with subtype 3b achieved SVR12, versus 89.3% (25 out of 28) non-cirrhotic patients with subtype 3b. Furthermore, the study reported that all patients with subtype 3b had a baseline NS5A RAVs, and most had both Ala30Lys and Leu31Met substitutions, consistent with in vitro studies (7), which suggests that the HCV subtype 3b is inherently resistant to current NS5A inhibitors. Therefore, patients with the HCV subtype 3b, presenting cirrhosis of the liver may have suboptimal responses to SOF/VEL treatments. Nonetheless, this should be further confirmed by future studies aiming at characterizing these genotypes and their responses to current treatments in detail. Fortunately, patients with HCV genotype 3b and cirrhosis were over-represented in the aforementioned study because of enrolment targets that enriched for these patients (5%), compared with the estimated prevalence of these patients in China (about 0.7%) (29) and these results cannot be directly applied to the general population. Again, more trials should be performed to assess the effectiveness of the SOF/VEL regimen in those countries. In our opinion, the global distribution and prevalence of HCV-genotype 3b have not been defined. However, it has been reported in recent articles that the estimated prevalence rates of HCV subtype 3b in Pakistan and in China are 8.20% and 7.06%, respectively, whereas the frequencies of GT3 cases are significantly higher in South Asia and China (30, 31). These results support the notion that upfront genotype testing may be unnecessary before treating HCV-GT3 patients with the SOF/VEL regimen, although the HCV subtype 3b may be suboptimal to it. We believe that testing the subtype of HCV is necessary for HCV-GT3 patients, who live in those areas where the prevalence rates of HC-GT3b are higher. It is recommended by the current Chinese medical guidelines that if the estimated prevalence rate of HCV subtype 3b is below 5%, an upfront genotype testing is unnecessary before treating HCV-GT3 patients with SOF/VEL. Otherwise, it appears necessary to perform further testing (32).
The SVR12 rates obtained by the HCV subgroups tested can be seen in Figure 3. In patients presenting compensated cirrhosis and GT3, the use of SOF/VEL yielded an SVR12 rate above 95% (96.3%). Specifically, our meta-analysis supports the guidelines recommended by the AASLD and shows that the addition of ribavirin to this regimen is unnecessary. A ribavirin-free regimen, which can avoid the adverse events (AEs) associated with ribavirin treatment and testing, greatly simplifies treatment and promotes the quality of life (QoL) of the patient. The same effectiveness of the SOF/VEL regimen was observed in HCV-GT3 patients with prior HCV treatment, with a SVR12 rate of 94.0%. Our meta-analysis supports the strategy to prescribe SOF/VEL without ribavirin for HCV-GT3 patients regardless of the compensated cirrhosis status and/or previous history of interferon-based regimens. Moreover, HCV-GT3 patients with a NS5A RAVs baseline presented a SVR12 rate of 88.1%, however this result should be interpreted with caution, given that there were insufficient samples, significant heterogeneities between the studies, and a lack of adequate data to assess the crucial risk of Y93H substitution. Another study showed that the addition of ribavirin to this regimen leads to slight increase in SVR12 rates in patients with HCV-GT3 and cirrhosis (10). Considering WHO’s simplified strategy for eliminating HCV, the SOF/VEL regimen plus ribavirin is a suboptimal treatment choice for these patients. Indeed, the SOF/VEL regimen is being successfully used for the elimination of HCV infection in Iceland (33).
As a meta-analysis for single group proportion, one of the limitations of our study is the absence of a control group, however the SVR12 rates found were high enough that a control group may appear to be less valuable. Another limitation of our study was a potential risk of publication bias for 10 out of the 11 studies, which were funded by Gilead Sciences, a major developer in this space. Although the funnel plot and Egger test did not support it, these results will need validation from more independent clinical trials, as well as real-world studies. The last limitation of our study was that our analysis did not assess critical risks affecting the effectiveness of the SOF/VEL regimen, which are the Y93H substitutions, co-infection with HIV, and intravenous drug use. These topics will need more investigations in order to be evaluated.
Compared to current pan-genotypic combination regimens, such as glecaprevir/pibrentasvir (Mavyret, Abbvie), and SOF/VEL/voxilaprevir (Vosevi, Gilead), the SOF/VEL regimen has multiple advantages, which are fewer contraindications, drug-drug interactions, and AEs. These potential benefits may improve patient adherence and reduce the cost of management, in turn, facilitating regimen efficiency. In particular, WHO has recommended intervention strategies for reaching HCV elimination targets by 2030, such as 90% of patients will be diagnosed, 80% will receive treatment, and 90% will be cured. Therefore, the single-tablet regimen consisting of SOF/VEL may be the better choice to include in these intervention strategies, given its efficacy, highly efficiency, well-tolerance, readiness of use (e.g., once daily for a uniform duration), independence of compensated cirrhosis status, as well as history of interferon-based regimen usage. Importantly, if a proportion of patients fail to respond to the SOF/VEL regimen or relapse, they could be successfully re-treated with SOF/VEL, in combination with voxilaprevir (34).
In summary, our meta-analysis show a high SVR12 rate for the single table regimen consisting of SOF/VEL in HCV-GT3 and recommends SOF/VEL without ribavirin for HCV-GT3 patients regardless of their compensated cirrhosis status and/or prior history of interferon-based regimen treatments. Meanwhile, other studies have shown that the HCV subtype 3b may be suboptimal for SOF/VEL treatment. Particularly, our results highlight the need for more clinical trials investigating the effectiveness of the SOF/VEL regimen versus other treatment options in patients with HCV subtype 3b, which is a less common subtype than HCV-GT3.