1. Background
Autoimmune liver disease (AILD) constitutes a group of chronic inflammatory diseases characterized by hyperglobulinemia, presence of auto-antibodies, and inflammation within the liver (1, 2). Primary Biliary Cirrhosis (PBC), Autoimmune Hepatitis (AIH), and Primary Sclerosing Cholangitis (PSC) are the classic types of AILD. Up to date, the etiology of AILD is not well understood. Besides genetic and interacting environmental factors, the dysregulation of immunological tolerance is likely to play an important role in the AILD pathogenic process (3-5).
Adenosine is a key immunosuppressive signal in microenvironments that is generated from the degradation of ATP after being catalyzed by CD39 and CD73. Adenosine promotes regulatory T cells (Treg) proliferation and activity (6). Treg cells, known as suppressor T cells, play an important role in the modulation of immune response and maintenance of immunological tolerance. Grant and Liberal reported that CD39 expression was decreased in Treg and T helper (Th) cells in AILD patients (7, 8). The decreased CD39 expression was associated with the impaired generation of adenosine. Thus, adenosine metabolism might be involved in AILD development. Adenosine deaminase is a key enzyme involved in the adenosine metabolism pathway, which catalyzes the deamination of adenosine (9). Adenosine deaminase plays an important role in the maturation and maintenance of immunological response (6, 10, 11). It has been found to be essential for lymphocytic proliferation and differentiation (12-14). As ADA is associated with the adenosine level and lymphocyte activity, the altered level of ADA may help in indicating immune disorders. Previous studies have shown that ADA activity significantly increases in multiple types of autoimmune diseases (15-19).
2. Objectives
Adenosine deaminase has two major isoenzymes, ADA1 and ADA2 (20). In this study, we investigated the serum activity of tADA and its isoenzymes in Chinese AILD patients, viral hepatitis patients, and healthy subjects. The main objective was to evaluate the changes in serum tADA, ADA1, and ADA2 activity in AILD patients.
3. Methods
3.1. Subjects and Sample Collection
The study was carried out between 2016 and 2018. In total, 50 AILD patients (19 AIH and 31 PBC patients) were recruited from the Department of Rheumatology and Immunology. Patients had not received any treatment before blood sampling. We also recruited 60 healthy subjects as the normal control group and 33 viral hepatitis patients as the disease control group. Viral hepatitis patients were recruited from the Infection Disease Department. The clinical characteristics of the patients and healthy subjects are listed in Table 1. Ethical approval was obtained from the Ethics Committee of Tangdu Hospital, Fourth Military Medical University (TDLL-20151209), Xi’an, China. This study used blood samples remaining after the medical examination and no additional blood was collected from patients. Waiver of informed consent would not affect the rights and interests of the subjects. Thus, informed consent was not required in this study.
| Clinical Parameter (Normal Interval) | AILD Patients (N = 50); Median (IQR) | Viral Hepatitis Patients (N = 33); Median (IQR) | Healthy Controls (N = 60); Median (IQR) | P Value (AILD vs. Healthy) | P Value (Viral Hepatitis vs. Healthy) | P Value (AILD vs. Viral hepatitis) |
|---|---|---|---|---|---|---|
| AST (15 - 46 U/L) | 59.5 (36.25 - 98) | 57 (26 - 67) | 24 (20 - 26) | 0.000 | 0.000 | 0.232 |
| ALT (11 - 66 U/L) | 52 (22.75 - 93.5) | 52 (20 - 93) | 21 (15 - 28) | 0.000 | 0.000 | 0.818 |
| ALP (40 - 150 U/L) | 220 (137.75 - 349.5) | 87 (65 - 122) | 81 (93 - 101) | 0.000 | 0.503 | 0.000 |
| TP (63 - 82 g/L) | 63.2 (58.8 - 69.5) | 59.7 (54.4 - 61.1) | 70.5 (67.65 - 73.30) | 0.000 | 0.000 | 0.007 |
| ALB (35 - 50 g/L) | 32.9 (30.58 - 35.4) | 33.1(30.9 - 35.4) | 42.60 (41.35 - 43.65) | 0.000 | 0.000 | 0.7694 |
| GLO (20 - 32 g/L) | 30.55 (27.45 - 34.22) | 23.6(21.8 - 27.5) | 28.10 (25.65 - 29.90) | 0.004 | 0.003 | 0.000 |
| ALB/GLO (1.5 - 2.5) | 1.1 (0.9 - 1.3) | 1.2 (1.4 - 1.5) | 1.5 (1.4 - 1.6) | 0.000 | 0.01 | 0.001 |
| TBIL (3 - 22 μmol/L) | 33.05 (19.13 - 49.75) | 39.2 (23.7 - 136.7) | 13.40 (11.30 - 16.85) | 0.000 | 0.000 | 0.178 |
| DBIL (0 - 5 μmol/L) | 18.15 (7.8 - 30.4) | 21 (10.7 - 85.7) | 4.9 (3.7 - 5.5) | 0.000 | 0.000 | 0.299 |
| IBIL (0 - 19 μmol/L) | 13.25 (9.88 - 22.5) | 18.2 (13.5 - 49.9) | 8.9 (7.25 - 11.45) | 0.000 | 0.000 | 0.028 |
| TBA (0 - 13 μmol/L) | 49.3 (16.6 - 135.6) | 71.25 (18.55 - 154) | 3.5 (2.2 - 5.1) | 0.000 | 0.000 | 0.668 |
3.2. Serological Assessment
Hepatic function indices, high-sensitive C reactive protein (hs-CRP), erythrocyte sedimentation rate (ESR), and autoantibodies were measured according to the standard test procedures in the clinical laboratory.
3.3. Measuring tADA and Its Isoenzymes Activity
As previously described (18), serum tADA activity was measured based on the enzymatic method with a diagnostic kit (Shanghai Kehua Bio-engineering, China), adapted to an automated biochemistry analyzer (Hitachi 7600, Japan). The serum ADA2 activity was measured in the presence of 0.1 mM erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA), which is a selective inhibitor of ADA1. The serum ADA1 activity was calculated by subtracting the ADA2 activity from tADA activity. The results were expressed as Unit per Liter (U/L).
3.4. Receiver Operating Characteristic Curve Analysis
The Receiver Operating Characteristic (ROC) curve analysis was applied by plotting sensitivity against 1-specificity for various levels of serum ADA activity. The optimal cutoff value was determined at the maximum value for the Youden index, which was defined as the difference between the true positive rate and the false positive rate [sensitivity - (1-specificity)].
3.5. Statistical Analysis
The serum ADA activities in this study did not conform to the normal distribution, and thus were expressed as median and Interquartile Range (IQR). The nonparametric Wilcoxon test was used to analyze the difference in ADA levels between patients and controls. The chi-square test and Fisher-exact test were used to analyze the differences in the abnormal rates between serum ADA activity and other hematological indices. A P value of < 0.05 was used to define statistical significance. All analyses were performed by R version 3.4.2.
4. Results
4.1. Serum tADA and Its Isoenzymes in AILD Patients and Healthy Subjects
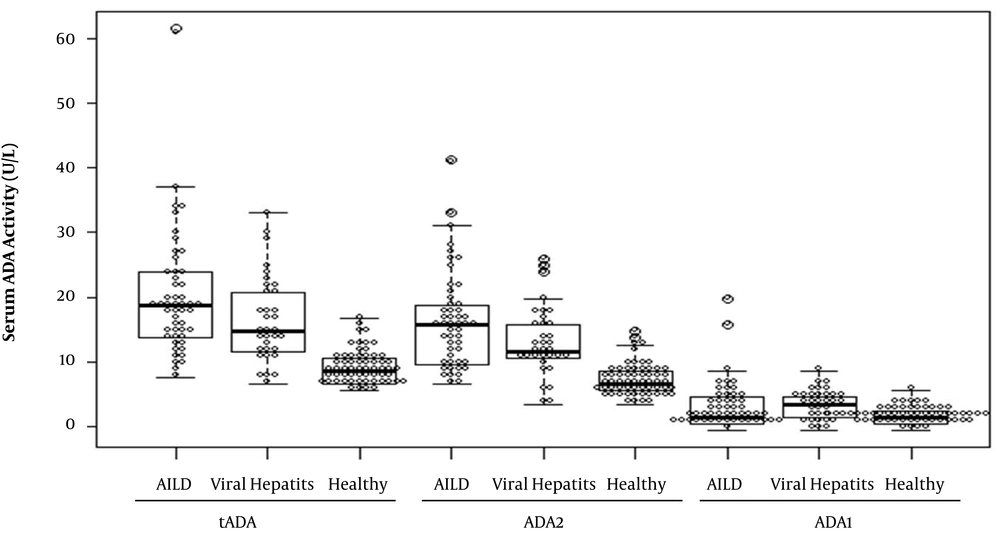
The serum tADA, ADA1, and ADA2 activities in AILD patients were 19 (IQR 14 - 24) U/L, 2 (IQR 1 - 5) U/L, and 16 (IQR 10 - 19) U/L, respectively (Table 2 and Figure 1). The serum tADA and ADA2 activities were significantly higher in AILD patients (P < 0.001) than in healthy controls and the serum ADA1 activity was significantly changed (P = 0.046). However, there were no significant differences in serum ADA levels between AILD and viral hepatitis patients (Table 2 and Figure 1). Because viral hepatitis patients could be easily identified by the virus infection test, serum ADA detection would be helpful in diagnosing AILD patients due to the significant difference of ADA levels between AILD and healthy subjects.
| AILD Patients (N = 50) | Viral Hepatitis Patients (N = 33) | Healthy Controls (N = 60) | P Value (AILD vs. Healthy) | P Value (Viral Hepatitis vs. Healthy) | P Value (AILD vs. Viral Hepatitis) | |
|---|---|---|---|---|---|---|
| Age | 56 (IQR 48 - 64) | 47 (34 - 53) | 55 .5 (IQR 51 - 60.25) | 0.369 | 0.000 | 0.000 |
| Female/Male | 39/11 | 6/27 | 44/16 | 0.571 | 0.000 | 0.000 |
| tADA (U/L) | 19 (IQR 14 - 24) | 15 (IQR 12 - 21) | 9 (IQR 7 - 11) | 0.000 | 0.000 | 0.049 |
| ADA1 (U/L) | 2 (IQR 1 - 4.5) | 4 (IQR 2 - 5) | 2 (IQR 1 - 3) | 0.046 | 0.001 | 0.29 |
| ADA2 (U/L) | 16 (IQR 10 - 19) | 12 (IQR 11 - 16) | 7 (IQR 6 - 9) | 0.000 | 0.000 | 0.054 |
Moreover, 19 AIH and 31 PBC patients were included in this study as the subtypes of AILD patients. The serum tADA, ADA1, and ADA2 levels were similar in AIH and PBC patients (Table 3). Thus, serum ADA activity was not correlated with AILD subtypes.
| AIH (N = 19) | PBC (N = 31) | P Value | |
|---|---|---|---|
| Age | 56 (IQR 42.5 - 63) | 56 (IQR 49 - 64.5) | 0.2887 |
| Female/male | 14/5 | 24/7 | 0.764 |
| tADA (U/L) | 19 (IQR 17.5 - 24.5) | 19 (IQR 14 - 23.5) | 0.378 |
| ADA1(U/L) | 2 (IQR 1 - 4) | 2 (IQR 1 - 5) | 0.744 |
| ADA2(U/L) | 16 (IQR 12.5 - 23) | 15 (10 - 18) | 0.218 |
4.2. Diagnostic Value of Serum tADA and ADA2 for AILD Patients
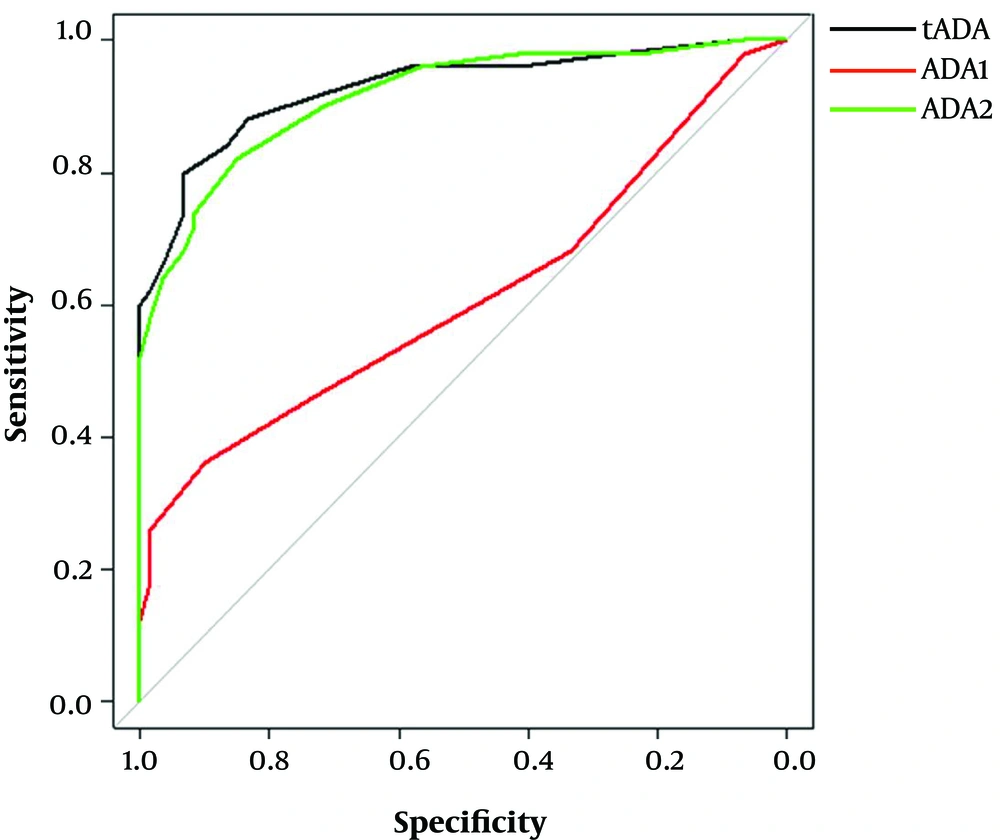
To further evaluate the potential clinical value of serum ADA activity in distinguishing AILD patients from healthy subjects, the ROC curve analysis was carried out to determine the cutoff value of tADA and ADA2 activity (Table 4 and Figure 2). Base on the ROC curve analysis, the optimal cutoff values of tADA and ADA2 activities were 11.5 and 9.5 U/L, respectively. At this level, the highest diagnostic performance of serum tADA (specificity: 83.3%; sensitivity: 88%) and ADA2 (specificity: 85.0%; sensitivity: 82%) was obtained. These results suggested that serum ADA activity could be an auxiliary index in distinguishing AILD patients from healthy subjects.
The ROC curve analysis of serum tADA, ADA1, and ADA2 activities for diagnosing AILD. Serum tADA (AUC = 0.928, 83.3% specificity and 88% sensitivity) and ADA2 (AUC = 0.916, 85% specificity and 82% sensitivity) showed the similar diagnostic efficacy (P = 0.3183), while ADA1 did not show any diagnostic value for AILD.
| Cutoff (U/L) | Youden index | Sensitivity (%) | Specificity (%) | AUC | 95% CI | P | |
|---|---|---|---|---|---|---|---|
| tADA | 11.5 | 0.713 | 88 | 83.3 | 0.928 | 0.878 - 0.978 | 0.000 |
| ADA1 | 2.5 | 0.173 | 44 | 73.3 | 0.612 | 0.505 - 0.7189 | 0.044 |
| ADA2 | 9.5 | 0.67 | 82 | 85 | 0.916 | 0.867 - 0.968 | 0.000 |
4.3. The Variation of Serum ADA Activity and Other Hematological Indices in AILD Patients
Multiple hematological indices (such as ESR, hs-CRP, auto-antibodies, and liver function) were measured. The variation rates of these indices in AILD patients are listed in Table 5, which shows most of them were less than 80%. To assess the potential application value of serum tADA and ADA2 activity in diagnosing AILD, we compared the difference of abnormal rates between serum ADA activity and other hematology indices. As shown in Table 5, the abnormal rate of serum tADA activity was 88%, which is higher than those of serum ADA2 activity (82%) and most other hematology indices. These data represent the potential value of serum ADA activity in diagnosing AILD patients.
| Markers | Abnormal Rate (%) | P (vs. tADA Activity) |
|---|---|---|
| tADA activity | 88 | |
| ADA2 activity | 82 | 0.578 |
| ESR | 70 | 0.065 |
| hs-CRP | 60 | 0.006 |
| AST | 68 | 0.028 |
| ALT | 38 | 0.000 |
| ALP | 66 | 0.028 |
| TP | 46 | 0.000 |
| ALB | 66 | 0.028 |
| GLO | 34 | 0.000 |
| ALB/GLO | 82 | 0.774 |
| TBIL | 64 | 0.009 |
| DBIL | 83.7 | 0.774 |
| ANA | 100 | 0.168 |
| Anti SS-A antibody | 25 | 0.000 |
| Anti SS-B antibody | 15 | 0.000 |
| AMA | 80 | 0.46 |
| CENPB | 20 | 0.000 |
5. Discussion
Autoimmune liver disease is considered a relatively rare disease. The AIH incidence ranges from 0.67 to 2.0 and the PBC incidence ranges from 0.33 to 5.8 per 100,000 population (21-23). Non-invasive laboratory biomarkers with better diagnostic performance are necessary for clinical practice. The destruction of immune homeostasis contributes to AILD development (24). Adenosine deaminase acts as an up-regulator of the immune response through catalyzing the hydrolysis of immunosuppressive signals, and plays an important role in maintaining the stability of human immunity. In this study, we investigated the serum ADA activity (tADA, ADA1, and ADA2) in AILD (19 AIH and 31 PBC), viral hepatitis, and healthy subjects. Notably, this was the first time that the levels of ADA isoenzymes were measured in AILD patients. Our data showed that tADA and ADA2 activity levels were significantly higher in AILD patients than in healthy subjects. Moreover, there was no significant difference in serum ADA activity between AIH and PBC patients. The ROC curve analysis showed that serum tADA and ADA2 activity detection would be helpful in distinguishing AILD from healthy subjects (tADA: 83.3% specificity and 88% sensitivity; ADA2: 85.0% specificity and 82% sensitivity). In a previous study, Torgutalp et al. (25) investigated the ADA activity in AIH patients from Hacettepe University. Their results showed that when the cutoff value of ADA was 25.25 U/L, the sensitivity and specificity were 84% and 88.9%, respectively, which are supported by the present study. However, the difference in the cutoff value for diagnosing might be due to the difference in the ADA determination method and race. Thus, these results indicated that for serum ADA detection, any clinical laboratory should establish its own reference intervals according to the actual situation.
Notably, there were no significant differences in serum ADA levels between AILD and viral hepatitis patients. This result means that ADA could not be used for differential diagnosis of AILD and viral hepatitis. Because viral hepatitis patients could be easily identified by the virus infection test, serum ADA detection would be still valuable for AILD patients, due to its significant difference between AILD and healthy subjects.
We compared the abnormal rate of ADA activity and other serological indices. The ESR and hs-CRP are conventional inflammatory indices usually used to evaluate the systemic inflammatory response. In this study, the abnormal rates of ESR and hs-CRP were 70% and 60% in AILD patients, respectively, which were both lower than the abnormal rate of serum tADA activity (88%). In this study, the abnormal rates of GLO and ALB/GLO ratio were 34% and 82%, respectively, which were both lower than that of tADA activity. The production and deposition of auto-antibodies are the important characteristics of AILD patients. Antinuclear Antibodies (ANA) are autoantibodies that bind to the contents (i.e. nucleic acid and protein) of cell nucleus, which are positive in serum of multiple autoimmune diseases, such as Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis (RA), Mixed Connective Tissue Disease (MCTD), and systemic sclerosis (26-30). Thus, ANA is considered a non-specific marker of autoimmune diseases. In this study, the positive rate of serum ANA was 100% in AILD patients. There are many subtypes of ANA, such as Centromerin B antibody (CENPB), anti-SS-A antibody, anti-SS-B antibody, anti-mitochondrial antibody (AMA), anti-ribosomal P protein antibody, etc., according to different recognized antigens. In AILD patients’ sera, the positive incidence of AMA antibody was 80%, which was higher than that of other auto-antibodies, while it was lower than that of tADA activity.
5.1. Conclusions
In conclusion, serum tADA and ADA2 activities were significantly higher in AILD patients. Particularly, our study showed that the abnormal rate of serum tADA and ADA2 activity were higher than those of other serological indices. Thus, serum ADA activity detection would be used for the clinical diagnosis of AILD, with lower costs and more sensitivity. Notably, any clinical laboratory should establish its own reference intervals according to the actual situation. Moreover, the difference in ADA1 and ADA2 activity suggested that ADA2 might play a more important role than ADA1 in AILD progression; however, while the mechanism needs further research.