1. Background
Liver Cirrhosis (LC) is an important cause of mortality worldwide, especially in China. The primary cause of cirrhosis is HBV infection. Approximately 3% of patients with compensated cirrhosis progress to Decompensated Cirrhosis (DeCi) each year (1). Decompensated cirrhosis is characterized by several complications, including ascites, hepatorenal syndrome, and upper gastrointestinal bleeding, leading to poor prognosis and a five-year survival rate of only 14% ~ 35% without any treatment (2, 3). Decompensated cirrhosis carries a poor prognosis, as the median survival time is about two years, and it imposes a heavy burden on health care costs, mainly due to the need for repeated hospital admissions (3, 4). The mortality rates of patients with DeCi treated at Intensive Care Units (ICUs) or hospitals range between 20% and 60% (5-8). At present, different scoring systems are used to assess the prognosis of patients to improve clinical management and reduce the high rate of mortality in these patients (8, 9). Therefore, the discovery of a marker with high practicability, especially in patients with DeCi at the ICU or hospital, is of crucial importance to guide therapeutic measures.
Serum urea is the end product of proteins. It is consistent with the level of protein metabolism and has been extensively reported in kidney disease, diabetes, pregnancy-induced hypertension, and fever following Transcatheter Arterial Embolization (TAE) (10-13). In a previous prospective study, Lei et al. suggested that serum urea could predict short-term outcomes of patients with hepatitis B virus-associated Acute-on-chronic Liver Failure (ACLF) (14). Mjasnikova et al. suggested that serum urea was associated with the Model for End-stage Liver Disease (MELD) score in HCV-induced liver cirrhosis, as well as with esophageal vein bleeding (15). However, there are currently a few accurate markers to predict long-term mortality after hospital admission of patients with DeCi.
2. Objectives
In the present study, we investigated serum urea as a predictor of 90 days and six months’ mortality in a cohort of DeCi patients.
3. Methods
3.1. Patient Selection
This single-center, observational prospective cohort study was conducted on patients admitted to the First Affiliated Hospital of Nanchang University between January 2013 and December 2017 who met the criteria of DeCi during their hospitalization. The ethics committee of the hospital reviewed and approved this study. All study participants or their legal guardians provided written informed consent before their enrollment in the study. All of the patients were given comprehensive supportive treatment after admission to the hospital and were followed up until death or for six months, whichever was earlier. Patients aged < 18 years, patients who were pregnant, and patients with cerebrovascular disease, cardiovascular disease, hematologic disorders, or renal failure were excluded from the study. The data from the medical records of the selected patients were input in the form of case reports and verified with the clinical data system in our hospital. All patients were treated following accepted recommendations and guidelines after admission to the hospital and they were followed up until death, loss to follow-up, or for six months (16, 17).
3.2. Definitions
In this study, DeCi was diagnosed by clinical, biochemical (e.g., low platelet count and detailed liver profile), and radiological (e.g., splenomegaly, coarse, nodular liver, and features of portal hypertension) performance, the presence of ascites, Hepatic Encephalopathy (HE), and/or endoscopic detection of esophageal or gastric varices or Portal Hypertensive Gastropathy (PHG), and liver biopsy results. Hepatorenal Syndrome (HRS) and ascites were diagnosed using the criteria proposed by the International Ascites Club and American Association for the Study of Liver Disease, respectively (18, 19). Moreover, ACLF was defined as patients with Acute Decompensation (AD) along with organ failure as per the Chronic Liver Failure-sequential Organ Failure Assessment scores.
3.3. Candidate Predictor Variables
Based on the clinical data in the medical record system, we collected patients’ demographics, clinical and laboratory parameters, and imaging findings. The laboratory variables included Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Total Bilirubin (TBil), Albumin (ALB), Prothrombin Time (PT), International Normalized Ratio (INR), White Blood Cell (WBC) count, Platelet (PLT), serum sodium (Na), Creatinine (Cr), serum urea, and oxygenation index (PO2/FiO2) within the first 24 h of diagnosis. In addition, the Child-Pugh score was calculated according to TBil, albumin, INR, ascites status, and degree of HE (20). The MELD score was calculated using the formula: 3.78 x Ln (TBil, μmol/L) + 11.2 x Ln (INR) + 9.57 x Ln (creatinine, μmol/L) + 6.43 × (constant for liver disease etiology, = 0 if cholestatic or alcoholic, 1 = otherwise) (21).
3.4. Statistical Analysis
The data are expressed according to the properties of variables. Continuous variables are presented as the median and interquartile range. Categorical variables are presented as frequency. Categorical variables were compared using the χ2 test and continuous variables were compared using the Mann-Whitney U test. The univariate and multivariate logistic regression analyses were employed to demonstrate the independent predictors of the mortality rate of patients with DeCi. All variables that were found to be associated with mortality (P < 0.10) in the univariate logistic regression analysis were included as candidate variables in a forward conditional stepwise logistic regression analysis to identify independent predictors of the prognosis of DeCi patients. The diagnostic accuracy of the prognostic variables was examined by Receiver Operating Characteristic (ROC) analysis using MedCalc version 15.2.1 statistical software (MedCalc, Ostend, Belgium). Statistical analyses were performed using SPSS version 16.0 software (SPSS Inc., Chicago, IL). All statistical tests were two-sided and a value of P < 0.05 was considered statistically significant.
4. Results
4.1. Baseline Characteristics
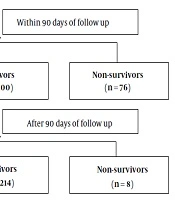
A total of 456 patients with DeCi during their hospitalization were included in this study. The flowchart is shown in Figure 1 and the baseline characteristics of this cohort are presented in Table 1. Patients’ age ranged from 21 to 89 years (median: 53.5 years) and 344 (75.4%) patients were male. Sixty-four (14.0%) patients received treatment at the ICU and 392 (86%) patients received treatment in the general ward. The presenting features of liver decompensation were as follows: 261 (57.2%) patients had ascites, 76 (16.7%) had HE, 398 (87.3%) had variceal bleeding, 21 (4.6%) had HRS, and six (1.3%) had spontaneous peritonitis. A total of 376 (82.5%) patients were followed up to 90 days and 298 (65.3%) patients were followed up for six months. A total of 76 (16.6%) and 84 (18.4%) patients died within 90 days and six months, respectively. The causes of death at six months were as follows: 15 (17.9%) patients had respiratory failure, 39 (46.4%) had a hemorrhagic shock, nine (10.7%) had hepatic encephalopathy, four (4.8%) had an infectious shock, five (5.9%) had the hepatorenal syndrome, four (4.8%) had acute-on-chronic liver failure, and eight (9.5%) were uncertain. The causes of death are summarized at 90 days and six months in Appendix 1 in Supplemental File.
| Variable | Patients with Decompensated Cirrhosis (N = 456) |
|---|---|
| Sex (male) | 344 (75.4) |
| Age | 53.5 (46 - 63.75) |
| Hospitalization days | 10 (6 - 12) |
| Intensive Care Unit | 64 (14.0) |
| Cause of liver cirrhosis | |
| Viral | 276 (60.5) |
| Alcoholic | 72 (15.8) |
| Combined alcoholic + viral | 37 (8.1) |
| Other | 28 (6.1) |
| Cryptogenic | 43 (9.4) |
| Cause of hospitalization | |
| Ascites | 3 (0.7) |
| Gastrointestinal hemorrhage | 407 (89.2) |
| Hepatic encephalopathy | 22 (4.8) |
| Infection | 24 (5.3) |
| Ascites degree | |
| No ascites | 195 (42.8) |
| One-degree ascites | 123 (27.0) |
| Two-degree ascites | 80 (17.5) |
| Three-degree ascites | 58 (12.7) |
| Acute renal failure | 20 (4.4) |
| Hepatocellular carcinoma | 56 (12.3) |
| Therapy | |
| Vasopressor support | 144 (31.6) |
| Mechanical ventilation | 27 (5.9) |
| Renal replacement therapy | 2 (4.4) |
| 90 days’ outcome | |
| Loss to follow-up | 80 (17.5) |
| Survival | 300 (65.8) |
| Non-survival | 76 (16.6) |
| Six months’ outcome | |
| Loss to follow-up | 158 (34.6) |
| Survival | 214 (46.9) |
| Non-survival | 84 (18.6) |
Characteristics of Patients in the DeCi Cohorta
4.2. Association Between Mortality and Clinical/Laboratory Characteristics
The clinical and laboratory characteristics of the patients are listed in Table 2. The DeCi patients were divided into non-surviving (n = 76) and surviving (n = 300) groups according to the 90 days’ survival outcomes. The DeCi patients were also divided into non-surviving (n = 84) and surviving (n = 214) groups according to the six-month survival outcomes. The non-surviving patients had higher ALT, AST, bilirubin, creatinine, urea, INR, PT, WBC, Child-Pugh score, and MELD score than surviving patients (P < 0.05). The non-surviving patients had lower albumin than surviving patients (P < 0.05). No significant differences were detected in platelet, serum Na, PO2/FiO2, and Mean Artery Pressure (MAP) (P > 0.05).
| Parameter | 90 Days | 6 Months | ||||
|---|---|---|---|---|---|---|
| Survivors (N = 300) | Non-survivors (N = 76) | P Value | Survivors (N = 214) | Non-survivors (N = 84) | P Value | |
| ALT, IU/L | 25 (17 - 38.25) | 27 (16 - 54.75) | 0.114 | 23 (17 - 36) | 26 (15 - 56) | 0.040 |
| AST, IU/L | 38 (26 - 57) | 61.5 (36.5 - 146.25) | 0.016 | 35.5 (26 - 53) | 57 (36 - 148) | 0.009 |
| Albumin, g/L | 29 (25.8 - 32) | 25.65 (22.8 - 29.275) | < 0.001 | 29.1 (25.925 - 32.1) | 25.7 (22.8 - 29.5) | < 0.001 |
| Bilirubin, mmol/L | 22.85 (14.85 - 38.7) | 27.1 (17.75 - 58.25) | 0.022 | 21.9 (14.3 - 37.475) | 29.5 (19.6 - 57.2) | 0.004 |
| Creatinine, mmol/L | 72.3 (59.525 - 88.875) | 92.55 (64.05 - 135.88) | < 0.001 | 71.45 (57.7 - 86.075) | 91.9 (63.4 - 132.6) | < 0.001 |
| Urea, mmol/L | 8.4 (6.3 - 10.85) | 12.2 (7.65 - 17.5) | < 0.001 | 8.5 (6.35 - 11.3) | 11.1 (7.5 - 17.225) | < 0.001 |
| INR | 1.3 (1.18 - 1.483) | 1.425 (1.27 - 1.75) | < 0.001 | 1.31 (1.19 - 1.487) | 1.4 (1.24 - 1.76) | < 0.001 |
| PT | 14.65 (13.175 - 16.425) | 15.6 (13.825 - 19.9) | < 0.001 | 14.7 (13.025 - 16.4) | 15.4 (13.7 - 19.9) | < 0.001 |
| Platelet, 109/L | 62 (40.75 - 92.25) | 69 (32.25 - 108) | 0.637 | 64 (41 - 92.75) | 70 (37 - 109) | 0.419 |
| WBC, 109/L | 6.33 (3.865 - 9.05) | 8.15 (4.715 - 13.63) | < 0.001 | 6.295 (3.805 - 9.432) | 7.75 (4.42 - 13.57) | 0.003 |
| Na, mmol/L | 138.95 (136 - 141) | 138.45 (134.85 - 142.75) | 0.939 | 138.6 (136 - 141.15) | 138.5 (135 - 142) | 0.967 |
| MAP, mmHg | 83 (78.33 - 89) | 82.833 (77.083 - 89) | 0.385 | 82.667 (79 - 88) | 83.667 (77.667 - 89.33) | 0.909 |
| PO2/FiO2, mmHg | 405.5 (349.75 - 477.75) | 396.5 (304.5 - 457.25) | 0.093 | 411 (349.25 - 480.5) | 391 (301 - 452) | 0.060 |
| Child-Pugh score | 8 (7 - 9) | 9 (8 - 10.5) | < 0.001 | 8 (7 - 9) | 9.5 (8 - 11) | < 0.001 |
| MELD score | 10.5 (9 - 14) | 15 (11 - 20) | < 0.001 | 10 (9 - 14) | 14 (11 - 19.5) | < 0.001 |
Association Between Clinical/Laboratory Characteristics and Mortality in DeCi Patientsa
4.3. Risk Factors Related to Prognosis of Patients with DeCi
Univariate logistic regression analysis showed that > 50 years of age, cryptogenic cirrhosis, ascites grade 3, HCC, ALT, AST, bilirubin, creatinine, urea, INR, PT, WBC, Child-Pugh score, and MELD score were risk factors for 90 days’ mortality in patients with DeCi (OR = 2.010, P = 0.014; OR = 2.465, P = 0.023; OR = 2.199, P = 0.026; OR = 2.272, P = 0.023; OR = 1.002, P = 0.023; OR = 1.115, P < 0.001; OR = 1.008, P = 0.023; OR = 1.013, P = 0.018; OR = 1.120, P < 0.001; OR = 3.996, P < 0.001; OR = 1.115, P < 0.001; OR = 1.055, P = 0.002; OR = 1.278, P < 0.001; and OR = 1.154, P < 0.001, respectively). However, albumin was a protective factor for 90 days’ mortality (OR = 0.871, P < 0.001). Multivariate logistic regression analysis identified that HCC, albumin, bilirubin, urea, and INR were related to 90 days’ prognosis (OR = 3.415, P = 0.003; OR = 0.899, P = 0.002; OR = 1.005, P = 0.042; OR = 1.084, P = 0.001; and OR = 2.010, P = 0.046, respectively). Univariate analysis of six months’ mortality found that > 50 years of age, cryptogenic cirrhosis, three-degree ascites, HCC, ALT, AST, albumin, bilirubin, creatinine, urea, INR, PT, WBC, Child-Pugh score, and MELD score were associated with prognosis (OR = 1.852, P = 0.033; OR = 2.296, P = 0.043; OR = 2.227, P = 0.025; OR = 1.977, P < 0.001; OR = 1.002, P = 0.012; OR = 0.997, P = 0.009; OR = 0.881, P ≤ 0.001; OR = 1.010, P = 0.001; OR = 1.012, P = 0.031; OR = 1.110, P ≤ 0.001; OR = 5.437, P<0.001; OR = 1.144, P < 0.001; OR = 1.059, P = 0.004; OR = 1.322, P < 0.001; and OR = 1.162, P < 0.001, respectively). Multivariate analysis showed that HCC, albumin, urea, and INR were independent factors for six months’ mortality (OR = 6.118, P = 0.001; OR = 0.893, P = 0.001; OR = 1.070, P = 0.009; and OR = 2.600, P = 0.031, respectively). Risk factors by univariate and multivariate analyses are summarized in Tables 3 and 4. The OR values adjusted for categorical variables are shown in Appendix 2 in Supplemental File.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Sex | 1.007 (0.569 - 1.783) | 0.980 | ||
| Age | ||||
| ≤ 50 | Reference | |||
| > 50 | 2.010 (1.150 - 3.513) | 0.014 | ||
| Cause of liver cirrhosis | ||||
| Viral | Reference | |||
| Alcoholic | 1.307 (0.647 - 2.639) | 0.455 | ||
| Combined alcoholic + viral | 1.417 (0.535 - 3.754) | 0.483 | ||
| Other | 1.741 (0.686 - 4.418) | 0.243 | ||
| Cryptogenic | 2.465 (1.134 - 5.361) | 0.023 | ||
| Cause of hospitalization | ||||
| Ascites | Reference | |||
| Gastrointestinal hemorrhage | 1.993 (0.178 - 22.304) | 0.576 | ||
| Hepatic encephalopathy | 2.833 (0.191 - 41.993) | 0.449 | ||
| Infection | 1.857 (0.065 - 11.256) | 0.907 | ||
| Ascites degree | ||||
| No ascites | Reference | |||
| One-degree ascites | 1.307 (0.647 - 2.639) | 0.433 | ||
| Two-degree ascites | 1.417 (0.535 - 3.754) | 0.589 | ||
| Three-degree ascites | 2.199 (1.100 - 4.395) | 0.026 | ||
| HCC | 2.272 (1.119 - 4.616) | 0.023 | 3.415 (1.551 - 7.521) | 0.003 |
| ALT | 1.002 (1.000 - 1.004) | 0.023 | ||
| AST | 1.115 (1.056 - 1.176) | < 0.001 | ||
| Albumin | 0.871 (0.821 - 0.924) | < 0.001 | 0.899 (0.840 - 0.961) | 0.002 |
| Bilirubin | 1.008 (1.004 - 1.012) | 0.023 | 1.005 (1.000 - 1.010) | 0.042 |
| Creatinine | 1.013 (1.007 - 1.019) | 0.018 | ||
| Urea | 1.120 (1.073 - 1.170) | < 0.001 | 1.084 (1.036 - 1.135) | 0.001 |
| INR | 3.996 (2.214 - 7.518) | < 0.001 | 2.010 (1.014 - 3.983) | 0.046 |
| PT | 1.115 (1.056 - 1.176) | < 0.001 | ||
| Platelets | 0.998 (0.994 - 1.001) | 0.144 | ||
| WBC | 1.055 (1.019 - 1.092) | 0.002 | ||
| Na | 1.015 (0.977 - 1.055) | 0.442 | ||
| MAP | 0.996 (0.991 - 1.001) | 0.142 | ||
| PO2/FiO2 | 0.998 (0.996 - 1.000) | 0.052 | ||
| Child-Pugh score | 1.278 (1.139 - 1.435) | < 0.001 | ||
| MELD score | 1.154 (1.000 - 1.210) | < 0.001 | ||
Univariate and Multivariate Analyses of Risk Factors Associated with Mortality at 90 Days
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P alue | |
| Sex | 0.877 (0.500 - 1.539) | 0.648 | ||
| Age | ||||
| ≤ 50 | Reference | |||
| > 50 | 1.852 (1.052 - 3.261) | 0.033 | ||
| Cause of liver cirrhosis | ||||
| Viral | Reference | |||
| Alcoholic | 1.148 (0.555 - 2.375) | 0.710 | ||
| Combined alcoholic+ viral | 1.060 (0.391 - 2.556) | 0.909 | ||
| Other | 1.330 (0.538 - 3.288) | 0.537 | ||
| Cryptogenic | 2.296 (1.026 - 5.138) | 0.043 | ||
| Cause of hospitalization | ||||
| Ascites | Reference | |||
| Gastrointestinal hemorrhage | 2.616 (0.162 - 42.381) | 0.498 | ||
| Hepatic encephalopathy | 2.750 (0.137 - 55.166) | 0.508 | ||
| Infection | 1.003 (0.053 - 18.915) | 0.997 | ||
| Ascites degree | ||||
| No ascites | Reference | |||
| One-degree ascites | 0.781 (0.400 - 1.527) | 0.470 | ||
| Two-degree ascites | 1.086 (0.510 - 2.312) | 0.830 | ||
| Three-degree ascites | 2.227 (1.107 - 4.483) | 0.025 | ||
| HCC | 1.977 (1.386 - 2.821) | < 0.001 | 6.118 (2.530 - 14.797) | <0.001 |
| ALT | 1.002 (1.001 - 1.004) | 0.012 | ||
| AST | 0.997 (0.997 - 0.999) | 0.009 | ||
| Albumin | 0.881 (0.832 - 0.933) | < 0.001 | 0.893 (0.834 - 0.956) | 0.001 |
| Bilirubin | 1.010 (1.004 - 1.016) | 0.001 | ||
| Creatinine | 1.012 (1.006 - 1.018) | 0.031 | ||
| Urea | 1.110 (1.052 - 1.149) | < 0.001 | 1.070 (1.017 - 1.126) | 0.009 |
| INR | 5.437 (2.563 - 11.536) | < 0.001 | 2.600 (1.091 - 6.196) | 0.031 |
| PT | 1.144 (1.074 - 1.220) | < 0.001 | ||
| Platelets | 1.003 (0.999 - 1.006) | 0.146 | ||
| WBC | 1.059 (1.018 - 1.101) | 0.004 | ||
| Na | 1.014 (0.981 - 1.048) | 0.417 | ||
| MAP | 1.004 (0.999 - 1.009) | 0.142 | ||
| PO2/FiO2 | 0.998 (0.996 - 1.000) | 0.054 | ||
| Child-Pugh score | 1.322 (1.169 - 1.495) | < 0.001 | ||
| MELD score | 1.162 (1.102 - 1.226) | < 0.001 | ||
Univariate and Multivariate Analyses of Risk Factors Associated with Mortality at Six Months
4.4. Predictive Value of Serum Urea for Prognosis of DeCi Patients
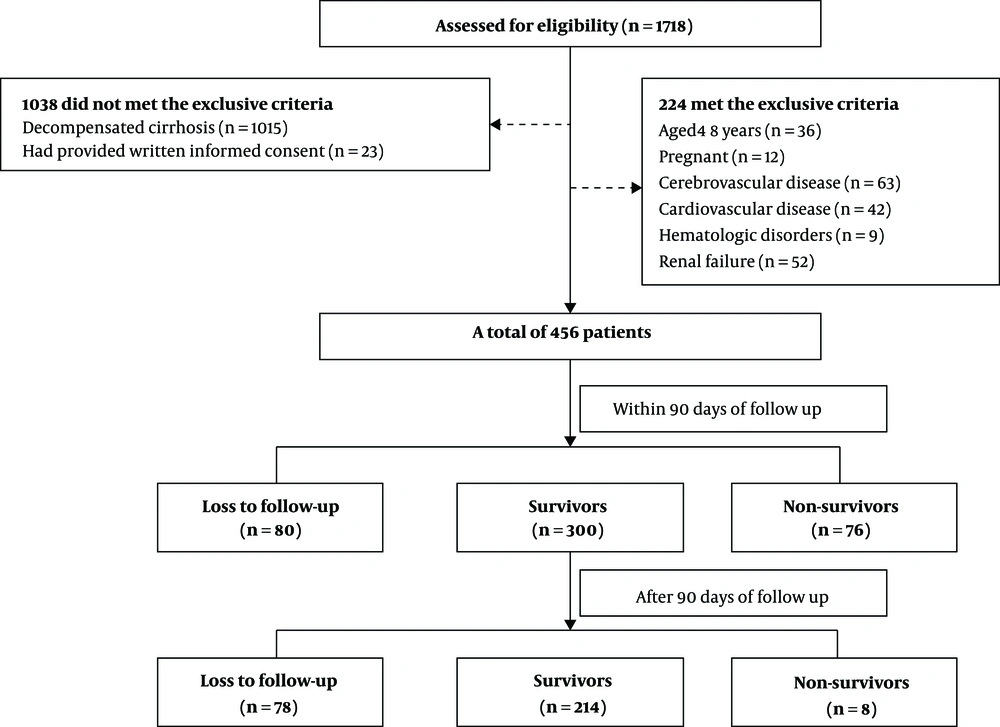
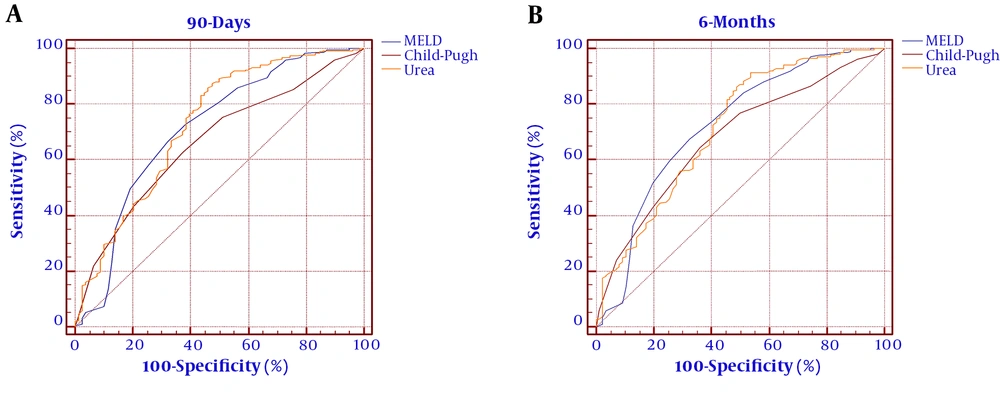
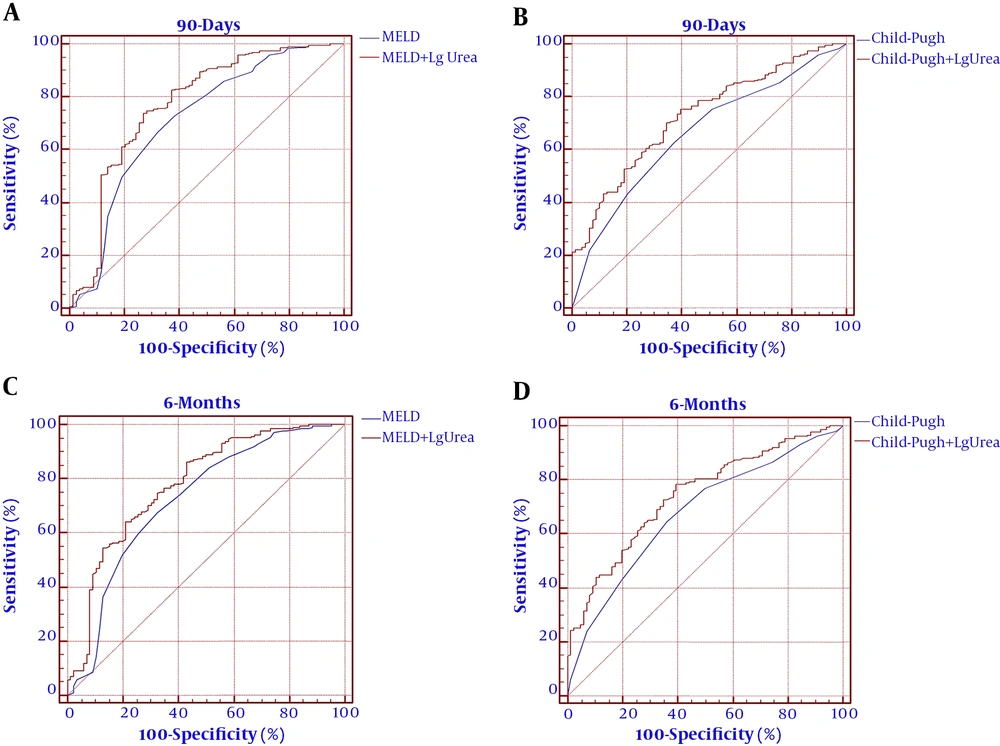
The ROC curves were established to evaluate the predicting efficacy of the MELD score, Child-Pugh score, and urea level. As shown in Figure 2 and Table 5, the MELD score Child-Pugh score, and urea level had predicting values for mortality at 90 days (AUROC = 0.711, 95% CI: 0.658 - 0.817; AUROC = 0.663, 95% CI: 0.563 - 0.720; and AUROC = 0.728, 95% CI: 0.672 - 0.856, respectively). The MELD score, Child-Pugh score, and serum urea showed significance in predicting mortality at six months (AUROC = 0.723, 95% CI: 0.667 - 0.831; AUROC = 0.679, 95% CI: 0.574 - 0.729; and AUROC = 0.715, 95% CI: 0.669 - 0.843, respectively). The cutoff value of urea for 90 days was 12.9 with a sensitivity of 87.54% and specificity of 52.56%. The cutoff value of urea for six months was 14 with a sensitivity of 91.43% and specificity of 46.51%. To improve the predictive value, new scores (MELD+ lg urea, Child-Pugh+ lg urea) were created by adding lg urea to the MELD score and Child-Pugh score. Comparing the AUROC at 90 days showed that the MELD+ lg urea score and Child-Pugh+ lg urea score were superior to the MELD score and Child-Pugh score, respectively (between-area difference = 0.069, 95% CI = 0.034-0.096, Z = 3.121, P < 0.001 and between-area difference = 0.075, 95% CI = 0.037 - 0.114, Z = 3.337, P < 0.001, respectively). Comparing the AUROC at six months showed that the MELD+ lg urea score and Child-Pugh+ lg urea score were superior to the MELD score and Child-Pugh score, respectively (between-area difference = 0.067, 95% CI = 0.022 - 0.093, Z = 3.174, P = 0.001 and between-area difference = 0.074, 95% CI = 0.038 - 0.112, Z = 3.441, P < 0.001, respectively). The ROC curves and comparison of prognostic scores are shown in Figure 3.
| Prognostic Score | ROC Area | Asymptotic Sig. | Cutoff point | Sensitivity (%) | Specificity (%) | PLV | NLV |
|---|---|---|---|---|---|---|---|
| 90 days’ mortality | |||||||
| Urea | 0.728 | < 0.0001 | 12.9 | 87.54 | 52.56 | 1.85 | 0.24 |
| Lg Urea | 0.728 | < 0.0001 | 1.11 | 87.54 | 52.56 | 1.85 | 0.24 |
| MELD score | 0.711 | < 0.0001 | 12 | 66.67 | 67.95 | 2.08 | 0.49 |
| Child-Pugh score | 0.663 | < 0.0001 | 8 | 62.96 | 62.82 | 1.69 | 0.59 |
| Six months’ mortality | |||||||
| Urea | 0.715 | < 0.0001 | 14 | 91.43 | 46.51 | 1.71 | 0.18 |
| Lg Urea | 0.715 | < 0.0001 | 1.15 | 91.43 | 46.51 | 1.71 | 0.18 |
| MELD score | 0.723 | < 0.0001 | 12 | 67.62 | 67.44 | 2.08 | 0.48 |
| Child-Pugh score | 0.679 | < 0.0001 | 8 | 64.76 | 63.95 | 1.80 | 0.55 |
Comparison of Prognostic Scores in Predicting 90-Day and Six-Month Mortality
5. Discussions
The prediction of prognosis is an important part of the management of hospitalized DeCi patients. The MELD score and Child-Pugh score are known as prognostic indicators for DeCi patients and are widely used in clinical practice, such as organ distribution standards for liver transplantation (22-24). As expected, the MELD score and Child-Pugh score can serve prognostic indicators for DeCi patients. However, the MELD score and Child-Pugh score also have some obvious deficiencies. The MELD score incorporates only three laboratory variables (TBil, INR, and creatinine) and is susceptible to diuretics, hemorrhage, and ascites (25-28). The Child-Pugh score contains two subjective parameters, i.e., ascites and encephalopathy, which may reduce the accuracy of assessment (29, 30). Due to population characteristics and observation time of the study, finding an optimal scoring standard is still a challenging issue. Most of the studies on the MELD score and the Child-Pugh score have concentrated in Western countries where the main cause of cirrhosis is alcoholic cirrhosis. Whether the MELD score is suitable for the Asian population needs more research. Therefore, it is meaningful to find a simple, practicable indicator to increase the predictive efficiency of the scores, especially in Asian countries.
We conducted a single-center, large sample, observational prospective analysis to evaluate simple laboratory parameters as predictors of mortality of DeCi patients. Consistent with a previous study on patients with liver cirrhosis, approximately 20% of the patients died within six months in the present study (31). The study was conducted for establishing the role of serum urea as a prognostic indicator for DeCi patients. We found that serum urea was significantly higher in non-surviving patients than in surviving patients (Table 2) and served an independent risk factor for long-term mortality (Tables 3 and 4). More importantly, our results indicated that serum urea could predict long-term mortality in DeCi patients (Table 5 and Figure 1) and the efficiency of the MELD score and the Child-Pugh score improved by adding lg urea (Figure 2). Serum urea is a biochemical test item that is often simultaneously detected with albumin, bilirubin, and transaminase indicators in clinical practice. A combination of lg urea with the MELD score and Child-Pugh score could increase the predictive efficiency without increasing testing costs.
The underlying mechanisms of how serum urea can predict the prognosis of patients with DeCi are not well established. Previous reports indicated that gastrointestinal hemorrhage would produce urea through liver metabolism (32). In Mjasnikova et al.’s study, serum urea was correlated with the MELD score in HCV-induced liver cirrhosis, as well as with esophageal vein bleeding (15). The energy consumption of hepatocellular carcinoma increases the decomposition of proteins, which, in turn, increases serum urea. In our study, hepatocellular carcinoma was an independent risk factor for mortality of DeCi patients. The correlation between liver cancer, serum urea, and patient prognosis remains to be further studied. Hepatorenal syndrome, which is a common complication of patients with DeCi, can also increase serum urea. In Gerbes et al.’s study, serum urea could be a valuable tool in patients with cirrhosis for early diagnosis of moderately impaired renal function although the diagnostic efficiency of serum urea was lower than that of serum cystatin C (33). The results of Lei et al.’s study indicated that serum urea was significantly associated with the short-term outcomes of hepatitis B virus-associated acute-on-chronic liver failure (14). Hence, we assume that the level of urea is a comprehensive marker of gastrointestinal hemorrhage, protein metabolism, and kidney function, which strongly impacts the prognosis of DeCi patients.
There were some limitations to the study. First, the present study was a single-center investigation in China and some patients were lost to follow-up, which carried bias in the participant selection and had some residual confounding factors due to unmeasured/unknown confounders. These findings need to be confirmed in large multicenter studies. Second, the serum urea level is affected by many factors, such as blood volume, drinking, infection, wounds, and steroid corticosteroid therapy. Lastly, we could not evaluate the predictive role of dynamic changes in serum urea, as the long-term changes in serum urea were not routinely measured in clinical practice.
In conclusion, many factors may be useful to predict the mortality of hospitalized DeCi patients, including MELD score and Child-Pugh score. Our results indicated that serum urea strongly and independently predicted long-term outcomes in DeCi patients. In terms of prognostic value, serum urea levels demonstrated a similar discriminatory power as the MELD score and Child-Pugh score and the predictive efficiency of the existing scores elevated by adding lg urea. From a clinical perspective, it is conducive to rapid diagnosis and timely treatment to reduce mortality from a clinical perspective.