1. Background
Egypt is one of the most affected countries by hepatitis C virus, currently employing a national plan for HCV control, together with the development of highly efficient direct acting antiviral drugs intended to treat over 250,000 infected individuals per year, thereby achieving a national chronic infection prevalence of < 2% by 2025 (1). Before this, Egypt was considered to have the highest prevalence of HCV in the world estimated to be 14.7% in 2008 (2). Later in 2015, a more conservative estimate of 10% was published (3). Considering this background and information about efforts done to treat the chronically infected, progress in the prevention of transmission should also be targeted.
The safety of blood and its components continues to be a global problem. Despite international and national guidelines, the hazard of blood-borne infections being transmitted via the blood is still a reality. This issue exacerbates blood shortage problem and draws worldwide attention to the significance of blood safety and accessibility to ensure global safe access to blood and its products (4).
To avoid Transfusion-Transmitted Infection (TTI), the current screening methods such as antibody-based detection methods e.g. ELISA or chemiluminescence are mainly used in most blood banks in Egypt, regarding the limitation of being serologically silent in window period. The use of HCV nucleic acid testing (NAT) aids in reducing the window period and identifying infection weeks to months before a detectable antibody response. Nucleic acid testing applied to donations in developed countries has become a routine procedure using different assays (5-7) and combination with serological testing leads to considerable risk reduction of TTI (8, 9); however, very low levels of viremia can be missed even by NAT (10).
Owing to logistics and cost reasons, NAT is usually performed in pools; in case of a positive pool, individual testing is performed and the identified positive unit is discarded and all negative units in the pool are used for transfusion (11).
2. Objectives
The objective of this study was to test the feasibility of pooling blood donations in screening for HCV by NAT, to propose a pooling scheme and to evaluate the impact of pooling on cost reduction.
3. Methods
3.1. Study Design
This cross-sectional study was carried out on blood donations received at Al-Shatby, and Medical Research Institute Blood Banks at Alexandria University Hospitals with a sample size of 12,036 blood donations.
The study included 2 sample groups:
- Group (1): A total of 10,020 blood donations proved to be seronegative for all markers of infectious diseases.
- Group (2): A total of 2,016 blood donations selected randomly to be tested blindly by NAT (without previous knowledge of their serology results).
After the initial interview and clinical examination of donors, demographic data were recorded, and all blood donations were tested at blood banks serological labs for the required essential screening tests (blood grouping, cross-matching, Syphilis serology, anti-HCV antibodies, HBs Antigen, and HIV antibodies) according to the Egyptian national standards for blood transfusion.
3.2. Sample Processing and Pooling
EDTA-containing blood samples were collected and centrifuged (10,000 rpm for 10 minutes), and plasma samples were used for pooling according to Wendel et al. method (12).
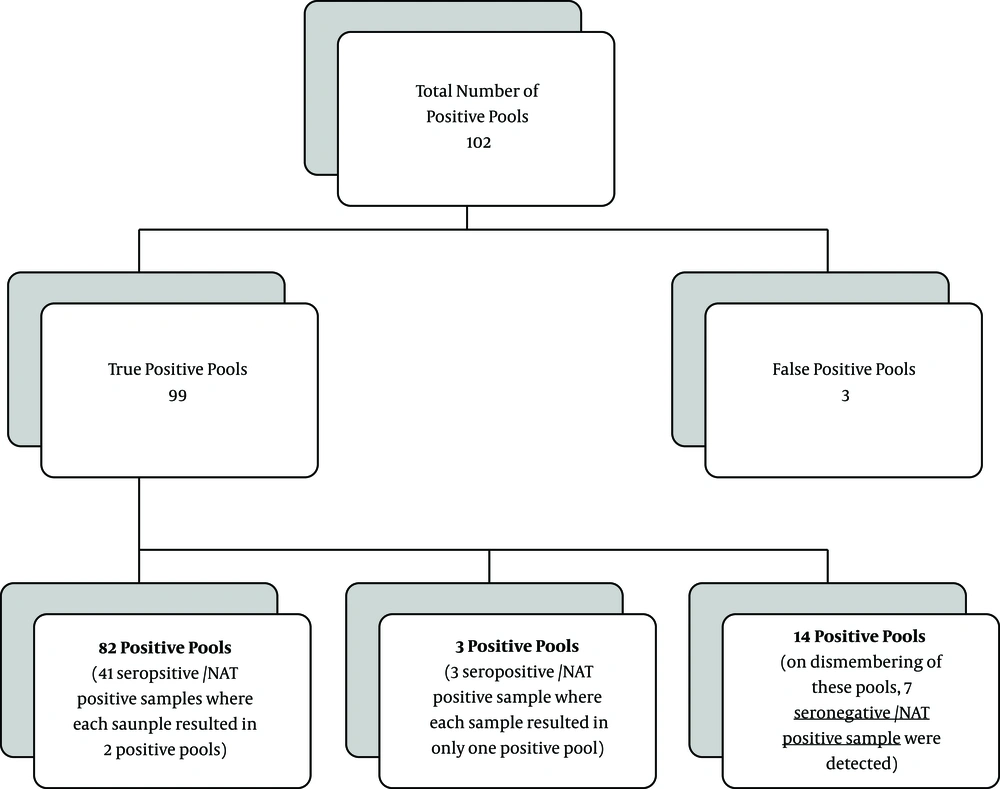
Samples were arranged twice bi-dimensionally (chessboard design) in mini-pools of 6x12 donations, each donation had a certain amount of plasma to a total pool size of 900 µL. For instance, in the pool containing six samples, each donation represented 150 µL to a total of 900 µL and in the pool of twelve, each donation represented 75 µL. By this format, every sample was represented in two pools allowing the positive sample to be easily identified, abolishing the need for breaking down the positive pools and retesting each donation separately (Figure 1). For verification, individual shared samples only were reassessed while quarantining all the donations sharing in the pool until confirmation was received.
3.3. Nucleic Acid Testing
Nucleic acid testing was carried out in the Molecular labs of Alexandria university hospital using Real-Time PCR (Roche Molecular Systems’ COBAS® AmpliPrep Instrument) according to manufacturer’s instructions. The test consists of three processes: (1) HCV RNA extraction by silica-based capture technique; (2) reverse transcription of the extracted RNA to produce complementary DNA, followed by (3) PCR amplification of cDNA and detection via the target-specific dual-labeled oligonucleotide detection probe. The accuracy of the results was assured through monitoring the assay against the incorporated internal controls as well as the individual positive and negative controls. The lower limit of quantitation was 15 IU/mL. The results below 15 IU/mL indicated that the result was valid but the concentration was below the limit of detection of the assay. This was further verified by testing the sensitivity of the assay used.
If any mini-pool was positive, the run was repeated individually for the plasma sample which was shared in the two reactive mini-pools in the chessboard format as a confirmatory test of the positivity of the shared sample, and consequently, that donation was excluded. Random individual samples (from un-pooled archived frozen plasma samples) were re-tested for HCV RNA by a different qPCR kit (Applied Biosystems, USA) for internal quality control. Moreover, other samples were re-tested by other institutions for external quality control.
3.4. Sensitivity of NAT
In order to identify the most appropriate bi-dimensional pool size, the sensitivity of COBAS® TaqMan® HCV assay on pooled plasma samples was performed using HCV negative pooled plasma samples of different sizes (pool of 3, pool of 4, pool of 6, and pool of 12) in chessboard format, spiked with known concentrations of RNA (from HCV positive samples and standards). The following known concentrations were used: 180, 90, 60, 30, 15 and 7.5 IU/mL in the different pool formats tested. Each concentration was tested ten times. After automated extraction and Real-Time PCR, the detection limit of the assay was determined as the least concentration at which all ten replicates gave a positive result in a bi-dimensional pool.
3.5. Statistical Analysis
Analysis of data was carried out using the Statistical Package for Social Sciences (SPSS Ver. 20 Chicago, IL, USA). Median and range were used to describe quantitative data, and the number and percent were used for the qualitative data. T-test was used to compare between the two groups (viremic and non-viremic). Quantitative variables Correlation was done using the Spearman coefficient. The receiver operating characteristics curve (ROC curve) analysis was done to determine the cutoff point with the maximum specificity and sensitivity. In all tests, P < 0.05 was considered statistically significant.
4. Results
Out of the 12,036 blood donors, 11,368 (94.44 %) were males and 668 (5.56 %) were females. Donors were in the age range of 18 - 60 years.
4.1. NAT Results
A 6x12 pool format was adopted for testing donations for HCV, based on testing the sensitivity of the available NAT in our labs on pooled samples, where it proved that a concentration of 30 IU/mL in a pool of six and a concentration of 90 IU/mL in a pool of 12 was the least detected. According to this pool format, group 1 donations (10020) were tested in 2,806 pools, while group 2 donations (2016) were tested in 504 pools, giving a total of 3,310 tested pools.
Of 3310 tested pools, 102 (3.08%) were NAT positive. Ninety-nine (99/3310; 2.99%) pools were true positive and three (3/3310; 0.09%) were false positive. Of 99 true positive pools, 82/99 pools represented 41 seropositive/NAT positive samples where the shared positive sample resulted in two positive pools (pool of six and pool of 12). Three other positive pools (3/99), which represented three seropositive/NAT positive samples, only the smaller size pool for each was positive (pool of six). The remaining 14 (14/99) true positive pools (representing 7 NAT positive samples) were all seronegative. Those seven seronegative/NAT positive samples were further individually confirmed in terms of positivity by NAT (Figure 2).
Three pools (3/102) were considered false positive as only one of the two pools in the chessboard format was positive and on further individual testing, none of the samples was NAT positive. All false positive pools had a low pool viral RNA level (< 15 IU/mL).
Regarding the group 1, of 10,020 seronegative donations, six (0.06%) seronegative/NAT positive donations were detected by the adopted pooling system. The six samples were individually retested as mentioned to confirm the result of the pooling method and were also retested serologically and proved to be negative for HCV antibodies.
After unfolding the serology results, of 2,016 blindly tested donations, 1,872 (92.9%) were seronegative, 47 (2.3%) were around signal to cut-off (S/CO) ratio, and 97 samples (4.8%) were HCV seropositive. Of the 97 seropositive samples, 44 (45.36 %) samples were positive for HCV RNA (seropositive/NAT positive) and 53 (54.64%) were negative (seropositive/NAT negative). All samples around S/CO ratio were NAT negative. Only one seronegative donation was NAT positive (the seventh seronegative/NAT positive sample). Thus out of the 11892 seronegative donations, 7 tested positive for HCV RNA (1:1698).
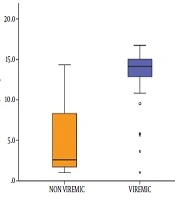
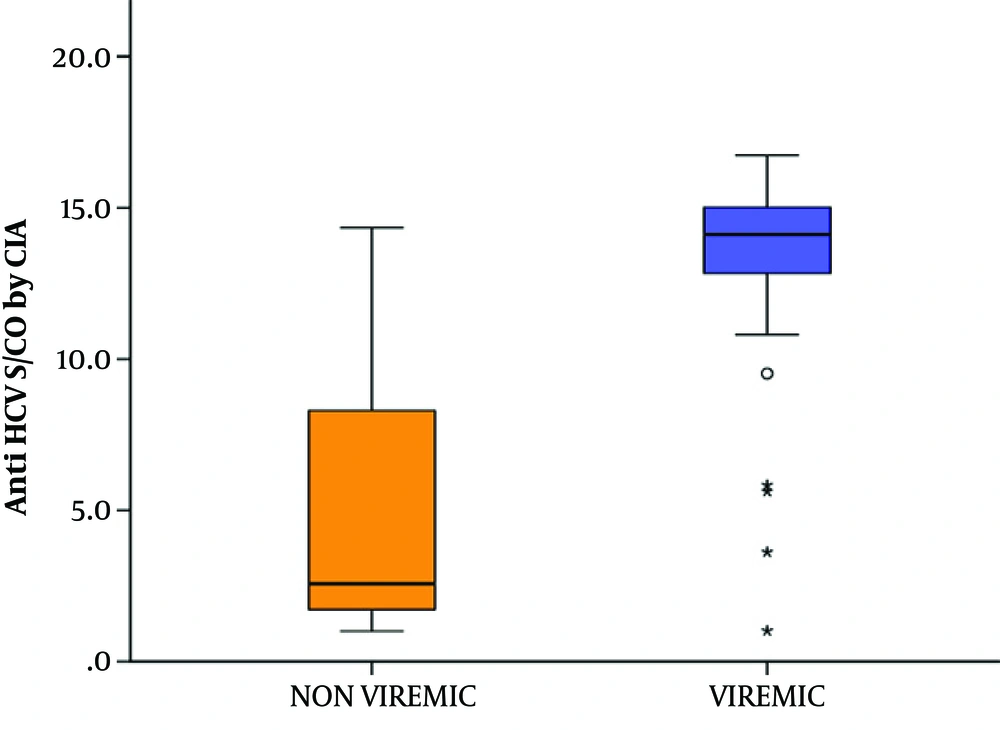
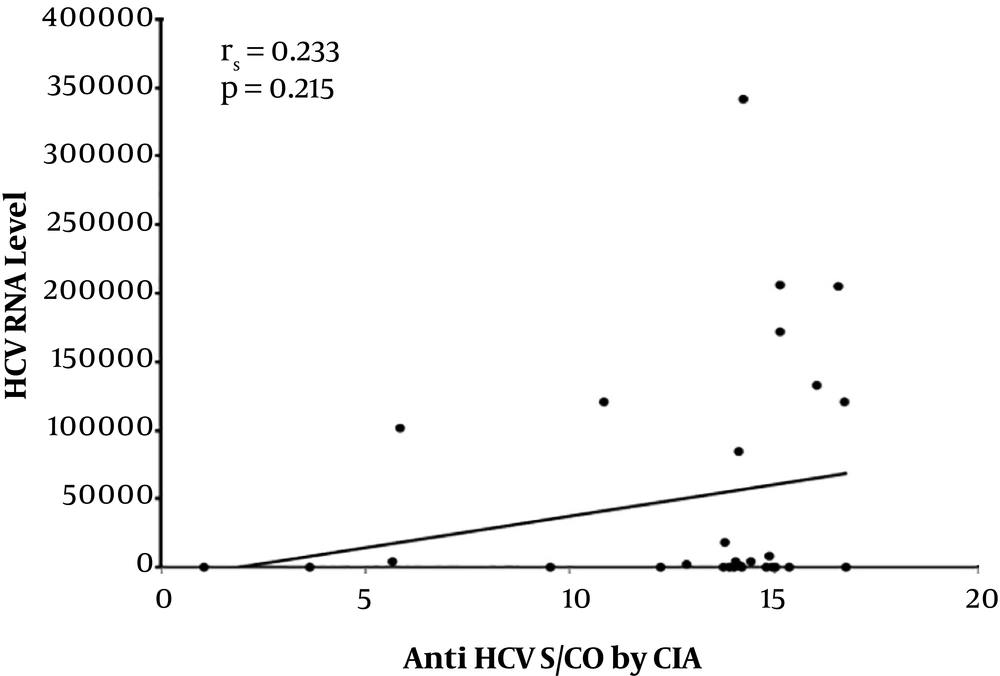
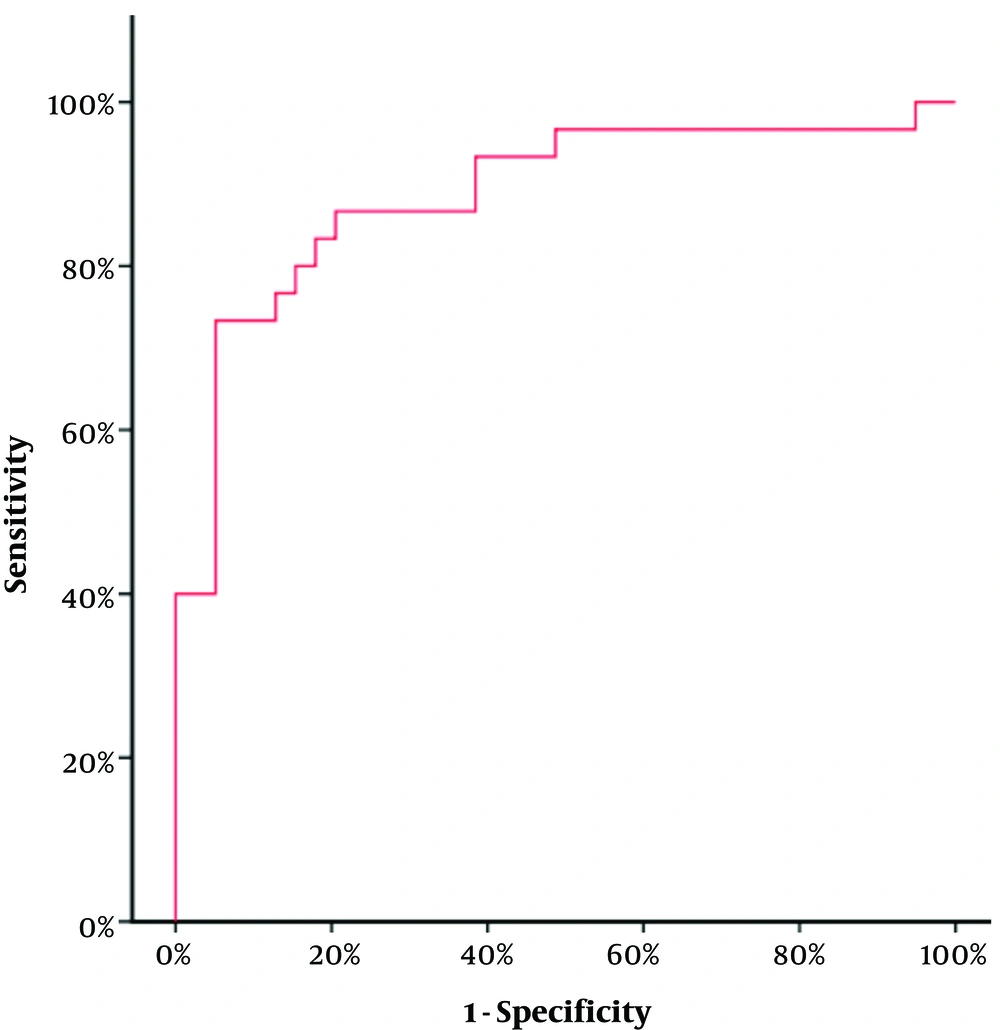
Concerning the 97 seropositive samples, a comparison between the viremic and non-viremic seropositive donors was done, according to anti-HCV chemiluminescence assay (CIA) S/CO ratio values. The mean CIA S/CO value of the non-viremic group was 5.30 ± 4.54 SD and the median value was 2.57. The mean CIA S/CO value of the viremic group was 12.91± 3.92 SD with a median value of 14.12. The viremic seropositive donors screened by CLIA were found to have a statistically significant higher CLIA S/CO ratio (Z = 5.483, P value < 0.001) (Figure 3). No correlation was found between anti-HCV CIA S/CO ratios and HCV RNA levels (r = 0.233, P value = 0.215) (Figure 4). To assess the diagnostic value of the CIA in determining the existence of viremia, a ROC curve were used, that pointed at an anti-HCV S/CO ratio cut-off of 13.44, the sensitivity, specificity, positive predictive value, and negative predictive value for HCV viremia included 73.57%, 94.87%, 91.67%, and 82.22%, respectively (Figure 5).
4.2. Effect of Pooling Scheme on Cost Reduction
Using the 6x12 pooling scheme reduced the cost to one-quarter of the price without affecting the sensitivity of the test, as by this format (6x12) every 72 donations are tested in only 18 pools. At the same time when donations were tested blindly by 6x12 pool format, all the viremic donations were detected, eliminating the need to breakdown the pool to individual sample testing as the chessboard format pinpoints the positive donation.
5. Discussion
Screening for HCV in Egyptian blood banks is mainly based on serological testing only despite the fact that Egypt is an HCV-endemic area. Only a few centers in Egypt have recently introduced NAT for the screening of blood donations (13).
In the present study, the HCV positive donations were 1:1698, which is much higher than studies done in the USA and Asia. NAT yield in the present study was even higher than the rate previously reported in similar studies conducted in Egypt (14, 15).
Wendel et al. (12) tested 139,678 donations by pooling method and compared NAT results of known serology results. Only 315 donations were seropositive/NAT reactive and no cases were found to be seronegative/NAT reactive. Sensitivity of their PCR method was 1,000 copies of HCV RNA/mL, which may have not been able to detect the window period low viral RNA load. Pools of 16 - 24 samples (mini-pools) have been used in the USA (16), while other countries (17, 18) implemented pools of 48 - 96 donations. The use of large pool sizes could be justified because of the decreased prevalence of HCV in these countries compared to Egypt.
In our study, three pools were false positive for HCV RNA (0.091%). Similarly, a false positive rate of 0.17% was reported in Japan (17), also, they reported 0.14% of initial false-positives in the Netherlands (19), and in a Croatian study, 0.04% were found to be false-positive (20). They stated that false-positive NAT results are often attributed to cross-contamination.
The non-viremic seropositive state may be explained by current active HCV infection, past exposure to HCV, or false-positive reactivity. Busch et al. have found that persons with active HCV infection can have negative HCV RNA in certain situations, as the level of antibodies surges during the acute phase, the HCV RNA titer drops (21). However, this is a transient finding in certain individuals during the acute phase where chronic infection can still develop. In addition, chronic HCV individuals have been known to have fluctuating and intermittent HCV RNA positivity (22, 23). Another possibility for these non-viremic seropositive donations is past exposure to HCV and resolved infection (24). Also, it has been noted by several studies that in areas with a low prevalence of HCV, even a highly specific assay might still fail to predict a positive case (25-27).
Our results revealed that the anti-HCV CIA S/CO ratio might be a parameter to predict viremia. However, we found no correlation between anti-HCV S/CO ratio and HCV RNA level. In a study by Seo et al. (28), they concluded that the anti-HCV S/CO ratio accurately predicts the presence of viremia with a cutoff value of 10.9 (sensitivity of 94.4% and specificity of 97.3%). They also found no correlation between RNA levels and the anti-HCV S/CO ratio.
Therefore, the significance of a single negative HCV RNA result is unknown in the lack of further clinical evidence, making serological evaluation a necessity, to cover situations where there is fluctuation in RNA levels (29). Hence, pooling donations for NAT testing in our setting in parallel with serology can prevent a considerable number of transfusion-transmitted HCV infections that can occur if one test only is used for screening. Moreover, this testing of pools, through detection of HCV-RNA during early stages of infection can reduce extensively the possibility of HCV transmission in anti-HCV negative (yet HCV positive) blood donations, thereby avoiding the deleterious effects of transmitting HCV while maintaining the cost-effectiveness as opposed to individual sample testing.
The NAT testing via pooling in the present study was capable of detecting seven positive donations, which were negative by serology and thus would have been released for use (30). Chronic HCV infection, is a principal cause of cirrhosis, liver failure and hepatocellular carcinoma, even if these complications do not develop, HCV diminishes the health-related quality of life (HRQOL), where the impact of infection seems to be most intense regarding physical and social aspects, general health and vitality. Given all these considerations, applying NAT testing to spare these recipients those hazards is definitely cost-effective. Other studies confirmed the cost-effectiveness of using NAT in blood banks (31, 32).
On the other hand, Jackson et al. (33) reported that although NAT can improve blood donation, the analysis displays that medical interventions are more cost-effective compared to NAT cost. Thus lowering NAT cost is a necessity to implement such a plan on the national level (34). Similarly, Al-Turaifi (35) found that the effectiveness of NAT for blood donors screening is an area of debate that a wide-national study is essential to weigh the safety and cost-effectiveness of implementing conventional and NAT assays for screening of blood donations.
5.1. Conclusions
Nucleic acid testing is an important screening technique, which should be adopted in Egypt to prevent further transmission of HCV by blood transfusion. Pooling donations by chessboard pool format is an effective way of reducing costs of NAT, especially with the limited health resources. Serology, besides not being able to detect window period cases, gives a high percentage of false-positive results. Therefore, combining NAT and serology might be considered a cost-effective addition in improving blood safety even in countries with limited resources. Further national studies are needed to evaluate the cost-effectiveness of NAT in Egypt regarding the residual risk of acquiring HCV infections in terms of cost-benefit and cost-utility analysis after receiving a blood transfusion when using serological screening alone and when using serology and mini-pool NAT screening in an attempt to set a regional protocol.