1. Background
Atrial septal defect (ASD) is a prevalent congenital heart disease in adults which is defined as a shunt from the left to the right side of the heart at the atrial level. Volume overload in the right heart influences the position of the interventricular septum, which can change the geometry of the left ventricle. The combination of ASD and different mitral valve diseases has been discussed in many studies. Some of these abnormalities are congenital, or primary, and others are secondary to hemodynamic effects of left to right shunt at atrial level. All these abnormalities could have significant hemodynamic effects on the left heart or pulmonary artery pressure (1-5). Awareness of the coexistence of the lesion and its effect on mitral valve regurgitation severity is vital for the diagnosis and optimal management of patients (6-9).
2. Objectives
However, there is inadequate data about the prevalence and different types of ASD associated with mitral valve abnormalities in the Iranian population. Hence, the present study evaluated the different types of ASD associated with mitral valve abnormalities and their hemodynamic effects on mitral valve insufficiency among adult Iranian patients.
3. Methods
In this retrospective study, we reevaluated 1,006 consecutive patients referred to the echocardiography ward of Rajaei Heart Center for the shunt study from March 2019 to February 2022. The study population included all patients with a confirmed diagnosis of ASD, but patients with poor echo views or without transesophageal echocardiography (TEE) or 3D study were excluded.
All transthoracic echocardiographic and transesophageal echocardiography studies were performed on Philips EPIQ devices, and all ultrasonography systems were equipped with 1 - 5 MHz transthoracic echocardiography (TTE) transducers and 2 - 7 MHz TEE transducers, 3D program, continuous wave, pulsed wave Doppler, and Color Doppler imaging. Patients received mild sedation with propofol and midazolam during the TEE studies. All echocardiographic examinations were recorded, and we reanalyzed the patient's data for further evaluation. All echo studies evaluated patients with 2D, Doppler, and 3D modalities. Analysis of LVEF was done with visual assessment and the Simpson biplane method. mitral regurgitation (MR) severity was analyzed by visual assessment and confirmed according to AHA 2020 guidelines of valvular heart disease with Doppler and PISA methods, including regurgitant volume, regurgitant fraction, and effective regurgitant orifice. All ASD sizes and anatomy of mitral valves were evaluated with 3D TEE. The ASD size and anatomy were evaluated with MPR and 3D zoom modalities of the 3D echo study. The MV anatomy was evaluated by 2D and 3D TTE and TEE, including full volume, 3D zoom, and mitral valve navigation (MVN) modalities of Philips EPIQ devices.
In this study, Peak Tricuspid Regurgitation Gradient (PTRG) with the addition of mean right atrium (RA) pressure was used to evaluate estimated pulmonary arterial pressure, and the value of more than 35 mmHg was defined as pulmonary hypertension (PH).
3.1. Statistical Analysis
Data were described as mean (± SD) for interval variables and frequency (percentage) for categorical variables. We compared data between subgroups via Mann-Whitney U/Kruskal-Wallis tests for interval variables, Pearson's chi-square test for nominal variables, and chi-square for trends test for ordinal variables. P-value < 0.05 was considered statistically significant. We performed statistical analyses by IBM SPSS Statistics 22 for Windows (IBM Inc., Armonk, NY).
All patients provided their informed consent before performing TEE. The Ethics Committee of Rajaei Heart Center approved this study with the ethical code of IR.RHC.REC.1400.104.
4. Results
In this study, we evaluated 1,006 patients. The mean age of the participants was 42.56 ± 13.82 years (range 15 to 85 years). The study population included 366 (36.4%) males and 640 (63.6%) females. ASD was more prevalent among females (the male-female ratio was 1:1.750) (Table 1).
All types of ASD were identified among 1,006 patients. The ostium secundum type was found in 893 (88.8%) cases, ostium primum in nine (0.9%) cases, sinus venosus SVC type in 91 (9%) cases, sinus venosus IVC type in four (0.4%) cases, and unroofed coronary sinus in nine (0.9%) cases (Table 1).
| Variables | ASD Type | Total | ||||
|---|---|---|---|---|---|---|
| Secundum | Primum | SVC Type | IVC type | Unroofed CS a | ||
| Total | 893 | 9 | 91 | 4 | 9 | 1006 |
| Gender | ||||||
| Male | 313 (35.1) | 4 (44.4) | 47 (51.6) | 0 (0.0) | 2 (22.2) | 366 (36.4) |
| Female | 580 (64.9) | 5 (55.6) | 44 (48.4) | 4 (100) | 7 (77.8) | 640 (63.6) |
a Coronary sinus
Secundum ASD was markedly prevalent among female patients (female to male ratio 1.85:1). The IVC-type sinus venosus ASD and unroofed coronary sinus were also significantly dominant in the female population (Table 1). Nevertheless, the gender distribution was equal for SVC-type sinus venosus and ostium primum ASD, although the frequency of patients in these groups was not enough to conclude.
In this study, there was no ventricular septal defect (VSD) simultaneously in SVC or IVC-type sinus venosus VSD, nor unroofed coronary sinus defect, but we diagnosed perimembranous VSD in 1% (nine cases) of secundum-type ASD and 66.7% (six cases) of primum type ASD. Also, we found partial anomalous pulmonary venous connection in three (0.3%) patients with ostium secundum-type ASD, 91 (100%) cases of SVC-type sinus venosus ASD, and two (22.2%) cases of the unroofed coronary sinus (Table 2).
| Variables | ASD Type | Total | ||||
|---|---|---|---|---|---|---|
| Secundum | Primum | SVC Type | IVC Type | Unroofed CS | ||
| PAPVC | ||||||
| Total | 3 (0.3) | 0 (0.0) | 91 (100) | 0 (0.0) | 2 (22.2) | 96 (9.5) |
| RUPV | 0 (0.0) | 0 (0.0) | 11 (12.1) | 0 (0.0) | 2 (22.2) | 13 (1.3) |
| RUPV and RMPV | 1 (0.1) | 0 (0.0) | 80 (87.9) | 0 (0.0) | 0 (0.0) | 81 (8.1) |
| LUPV | 2 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.2) |
| VSD | 9 (1.0) | 6 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 15 (1.5) |
Abbreviations: RUPV, right upper pulmonary vein; RMPV, right middle pulmonary vein; PAPVC, partial anomalous pulmonary vein connection; LUPV, left upper pulmonary vein.
We found mitral valve abnormalities in 203 (21.1%) cases. Most mitral valve abnormalities were found in secundum-type ASD (details of MV abnormality summarized in Table 3).
| Variables | ASD Types | Total | ||||
|---|---|---|---|---|---|---|
| Secundum | Primum | SVC Type | IVC Type | Unroofed CS | ||
| Total ASD | 893 | 9 | 91 | 4 | 9 | 1006 |
| MV anomaly | ||||||
| Total MV anomaly | 176 (19.7) | 9 (100) | 12 (13.1) | 2 (50) | 4 (44.4) | 203 (20.1) |
| AMVL prolapse | 10 (1.1) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 11 (1.1) |
| PMVL prolapse | 7 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (0.7) |
| Both MVLs prolapse | 108 (12.1) | 0 (0.0) | 9 (9.9) | 2 (50) | 0 (0.0) | 119 (11.8) |
| Both MVLs prolapse and MAD | 2 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.2) |
| Prolaptic AMVL with hypoplastic PMVL | 33 (3.7) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 2 (22.2) | 36 (3.6) |
| Total prolaptic lesion | 160 (17.9) | 0 (0.0) | 11 (12.1) | 2 (50) | 2 (22.2) | 175 (17.4) |
| Hypoplastic PMVL | 4 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) | 5 (0.5) |
| Rheumatic MS | 12 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 (1.2) |
| AMVL cleft | 0 (0.0) | 9 (100) | 0 (0.0) | 0 (0.0) | 1 (11.1) | 10 (1.0) |
| DOMV | 0 (0.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
Abbreviations: AMVL, anterior mitral valve leaflet; PMVL, posterior mitral valve leaflet; MAD, mitral annular disjunction; MS, mitral stenosis; DOMV, double orifice mitral valve.
The most common mitral valve abnormalities in all ASD groups was prolaptic involvement of mitral valve. (175 or 17.4% of total patients). Prolapse of the mitral valve was diagnosed with 2D TTE in the parasternal long axis, defined as mitral valve displacement more than 2 mm above mitral annulus (1), confirmed by 2D TEE and 3D TEE with 3D zoom and MVN analysis modalities. One of the subgroups of prolaptic lesion was prolaptic anterior mitral valve leaflet (AMVL) in combination with hypoplastic posterior mitral valve leaflet (PMVL), which is not well described in previous studies. However, this diagnosis greatly impacts selecting the closure type of defect. In this study, 3.6% of the population had this anomaly, which will be described more in the discussion. The other anomalies found among the study population were rheumatic mitral valve stenosis, cleft of AMVL, and double orifice mitral valve (DOMV).
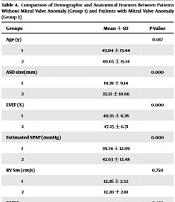
The size of ASD, estimated SPAP, and age of patients with mitral valve anomaly (group 2) were statistically significantly higher than those of patients without MV anomaly (group 1) (P-value < 0.05). Also, LVEF was lower in group 2 (Table 4). There was no significant difference in RV function (Sm and TAPSE) between these two groups.
| Groups | Mean ± SD | P-Value |
|---|---|---|
| Age (y) | 0.017 | |
| 1 | 43.04 ± 13.44 | |
| 2 | 40.65 ± 15.14 | |
| ASD size(mm) | 0.000 | |
| 1 | 19.78 ± 9.14 | |
| 2 | 25.12 ± 10.66 | |
| LVEF (%) | 0.000 | |
| 1 | 49.35 ± 6.76 | |
| 2 | 47.25 ± 6.71 | |
| Estimated SPAP (mmHg) | 0.000 | |
| 1 | 39.74 ± 12.99 | |
| 2 | 42.63 ± 12.48 | |
| RV Sm (cm/s) | 0.750 | |
| 1 | 12.26 ± 2.52 | |
| 2 | 12.20 ± 2.81 | |
| TAPSE | 0.458 | |
| 1 | 21.83 ± 4.49 | |
| 2 | 21.50 ± 5.00 |
Probable PH, measured by peak TR gradient with adding estimated RA pressure, was significantly higher in patients with MV anomaly (68.7% vs. 53.6%). Also, significant mitral valve regurgitation (defined as moderate MR or more) was significantly higher among patients with MV anomaly (20.3% vs. 4.1%) (Table 5).
| Group 1 | Group 2 | P-Value | |
|---|---|---|---|
| Probable PH (SPAP > 35 mmHg) | 53.6% | 68.7% | 0.00 |
| Significant MR a | 4.1% | 20.3% | 0.00 |
a MR severity of moderate intensity or higher.
5. Discussion
This retrospective study was performed to determine the frequencies of different types of ASD, the prevalence of mitral valve abnormalities, and their hemodynamic consequences associated with ASD in the adult population (5, 10-15).
In this study, the ratio of different morphological types of ASD was similar to that of the previous study, except for ostium primum, which was lower than in a previous study (about 1% vs. 10%) (10); it could be due to better screening and diagnosis of congenital heart disease during childhood in recent years.
Prolapse of the mitral valve was diagnosed with 2D TTE in the parasternal long axis, defined as mitral valve displacement more than 2 mm above mitral annulus (1), confirmed by 2D TEE and 3D TEE with 3D zoom and MVN analysis modalities.
The prevalence of isolated mitral valve prolapse is 2.5 - 5 % in the general population (6, 7, 15). According to previous studies, the prevalence of this abnormality is higher in women than in men (7). Based on the mechanism of prolapse, isolated MVP is divided into functional and anatomical groups. Characteristics of anatomical MVP are chorda elongation and leaflet elongation (6). Functional MVP is the result of hemodynamic changes in the LV cavity (i.e., dehydration, volume load, etc.). Furthermore, in some of patients with MVP in association with ASD, mitral regurgitation is functional and alleviated after repair of the atrial defect. On the contrary, in cases of anatomical change of mitral valve leaflets and apparatus, repair of ASD and resolution of left to right shunt in the atrial level can lead to increase in the severity of mitral regurgitation. Therefore, it is crucial to diagnose mitral valve prolapse mechanism, associated mitral valve regurgitation and hemodynamic consequences of it before planning for surgical or device closure of ASD (16-19).
Furugen et al, mentioned that based on a 3D study, volume overload in the right side of the heart, RV dilation, left-side shift of the inter-ventricular septum, and finally, the inward shift of the LV papillary muscle leads to shortening of the inter-papillary distance and redundant chordae tendinea. These are the geometric mechanisms of MVP in ASD patients, which is expected to improve after ASD closure (2, 20).
In this study, we found 17.4% prolaptic lesions among the patients, which was higher than in the normal population and more prevalent among secundum-type ASD. Mitral valve regurgitation and estimated Systolic Pulmonary Artery Pressure (SPAP) were higher among these patients than in patients without mitral valve abnormality (P-value < 0.05%), which could be due to MR severity and hemodynamic effects of MR on the pulmonary vasculature.
One of the subgroups of MVP in this study was the prolapse of the anterior mitral valve leaflet (AMVL) in combination with the hypoplastic posterior mitral valve leaflet (PMVL), which was not described well in previous studies. We found that hypoplastic PMVL is defined when the length of PMVL is less than 10 mm in 2D TTE or TEE, which can be confirmed with 3D echocardiography with 3D zoom or MVN modalities. Hypoplasia of the PMVL is an abnormal condition mainly diagnosed in childhood with symptoms of mitral regurgitation, even though in the milder degrees of valvular regurgitation, patients remain asymptomatic until late adulthood.
Pourafkari et al. mentioned a few literature reviews about hypoplastic PMVL in the adult population in the world because many of them become symptomatic due to severe MR during infancy (21). Thus, there is a lack of evidence in the literature on the frequency of this MV abnormality in the adult population.
Hypoplastic PMVL with or without prolaptic AMVL can be seen solely or associated with other cardiac conditions. Secundum ASD is the most common congenital defect associated with hypoplastic PMVL in the literature. When there is significant mitral regurgitation in these patients, transcatheter methods for ASD closure are not suitable, and patients need mitral valve repair or replacement in combination with surgical ASD closure (4, 22, 23).
In this study, 36 (3.6%) of total cases were diagnosed with this abnormality, which was more prevalent among secundum-type ASD (33 cases or 3.7%). Also, we found isolated hypoplastic PMVL in five (0.5%) patients, which was more prevalent in secundum-type ASD (4 cases or 0.4%). One of the reasons for the significant prevalence of this anomaly in this study could be due to the left to right shunt in the atrial level that alleviated the hemodynamic effect of mitral regurgitation, and patients became symptomatic later during adulthood.
Double-orifice mitral valve (DOMV) is a rare anomaly. It is mainly observed in patients with endocardial cushion defects, although it can be either an isolated defect or in association with other cardiac conditions. Greenfield first described the Double-orifice mitral valve in 1876 (8). We evaluated all patients for this anomaly by 2D TTE in parasternal short axis view at the level of the mitral valve and 3D TEE of MV by 3D zoom modalities. This malformation could lead to both mitral stenosis and, very rarely, mitral insufficiency, but this anomaly has no hemodynamic disturbance in most patients. In many reported cases, DOMV is seen as an isolated lesion (8). The frequency of this anomaly in the general population is not well described in previous studies. In this study, the prevalence of DOMV was 0.1%, seen only in one SVC-type sinus venosus, and it did not have a significant hemodynamic sequence (stenosis or regurgitation).
Lutembacher described atrial septal defect and simultaneous rheumatic mitral valve disease in 1916 (15). He described a patient with interatrial communication of the foramen oval type shunt and mitral stenosis, presumably of rheumatic origin, named "Lutembacher syndrome" (15, 24).
Aminde et al. found that the prevalence of rheumatic MS was up to 4% in patients with ASD, while the incidence of ASD in patients with rheumatic MS was 0.6-0.7% (25).
In this study, we diagnosed rheumatic MS by 2D TTE by thickening and doming appearance of MV in association with commissural fusion and chorda thickening confirmed by 2D and 3D TEE with 3D zoomed and MPR modalities. The frequency of rheumatic MS was 12 (1.2%) in all study populations, and all were among secundum-type ASD (Lutembacher syndrome). The lower prevalence of rheumatic MS in association with ASD compared to the previous studies could be due to better medical screening and resources in the current era.
The last mitral valve abnormality diagnosed in this study was cleft on AMVL. Clefts of the mitral valve leaflet are an integral component of ostium primum ASD. A cleft is regularly present in this anomaly, but it can be diagnosed solely (26). In this study, the cleft of MV has been diagnosed with 2D TTE and TEE and confirmed with 3D TEE with 3D zoom and MVN modalities. We found that 10 (1%) of all cases had the cleft of AMVL, and the majority of them (nine of all cases) were associated with primum ASD, which was similar to previous studies (10).
The gold standard of PH is diagnosis based on right heart catheterization, but in the present study, we evaluated the probability of pulmonary hypertension by echocardiography, measured by peak TR gradient with adding of estimated RA pressure. PH is defined as SPAP of more than 35 mmHg, which was detected in 68.7% of patients with mitral valve abnormalities; it was significantly higher than in patients without MV abnormality (53.6%, P-value = 0.05). Diagnosis of the milder degree of pulmonary artery hypertension is not unusual in many patients with ASD, which could be a consequence of increasing the patient's age or pulmonary vascular disease, but hemodynamic consequences of MR severity also have a great impression on SPAP.
In this study, MR severity was higher in patients with MV anomaly. Significant MR, defined as mitral regurgitation of moderate intensity or more based on the latest AHA valvular heart disease guideline (2020) and using the PISA method and calculation of MR regurgitant volume, MR regurgitant fraction, and effective regurgitant orifice (4, 27), was seen in 20.3% of patients with MV abnormalities versus 4.1% of patients without abnormalities. Patients with MV abnormalities had lower LVEF, and the mean LVEF was 2.1% lower among these patients.
These findings are important in planning for surgical or device closure of defects. So it is essential to recognize MV abnormality associated with atrial septal defect and evaluate the hemodynamic consequence of these anomalies before planning for any intervention.
5.1. Conclusions
Significant associations exist between mitral valve abnormalities and atrial septal defects, and these abnormalities have significant hemodynamic consequences. Hence, evaluations of these abnormalities and their hemodynamic effects are necessary before planning for intervention.
5.2. Limitations
We had some limitations in this study, including poor TTE or TEE views, unmeasurable peak TR gradient for estimation of SPAP by echocardiography, and lack of 3D studies for reanalysis of ASD or MV anatomies.