1. Background
Sudden cardiac death (SCD) is a significant cause of mortality as it accounts for about half of all cardiovascular deaths. This condition primarily results from ventricular tachycardia (VT) and/or Ventricular Fibrillation (VF) (1, 2). Prophylactic insertion of an implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy with a defibrillator (CRT-D) have reduced the mortality and morbidity in patients with a history of VT (as secondary prevention), and those at high risk for these fatal arrhythmias (as primary prevention) (2-4) since the first implantation in humans by Merowski in 1980 (5, 6).
Anti-tachycardia pacing (ATP) modalities have been programmed to terminate VT/VF episodes by ICD devices prior to the delivery of an electrical shock. Anti-tachycardia pacing is much more tolerable compared to frequent electrical shocks (7-10), which are unpleasant experiences associated with anxiety and adverse effects on the patient's quality of life (11-13). Shocks are suspected to be proarrhythmic (14) and seemingly increase the heart failure and mortality rate compared with ATP-treated arrhythmias (15, 16).
Various studies have shown ATP success rates of 60 - 90%, and many ATP episodes still fail to terminate tachyarrhythmia (17). Each episode of ATP therapy delays the delivery of more effective electrical shocks, prolongs hemodynamic instability, and causes loss of consciousness following pump failure during fatal tachyarrhythmia (18). Therefore, it is essential to explore and detect all contributing factors that enhance the ATP success rate to minimize the delay in the termination of VT episodes caused by failure of ATP therapies and the unnecessary subsequent electrical shocks.
2. Objectives
In this study, we investigated these factors in a study population.
3. Methods
3.1. Patient Selection
The current study was conducted on patients with a Medtronic ICD device implanted in a background of ischemic or dilated cardiomyopathy (ICM or DCM). They were referred to an arrhythmia clinic for regular device analysis from January to November 2019. None of the cases had a history of hypertrophic cardiomyopathy, channelopathies (long QT syndrome, Brugada syndrome, for instance), or arrhythmogenic right ventricular dysplasia. In all the included patients, the ICD device had been implanted for more than a year, and the RV lead was located at the right ventricle apex and the generator in the left subclavicular space. The configuration of far-field EGM was coil to can for all devices. All devices were programmed with at least two detection zones in all patients, and the first programmed therapy in the VT and FVT zone was Burst ATP with eight pulses. ATP therapies were delivered at maximum voltage and pulse duration.
3.2. Intracardiac Electrogram (EGM) Data Analysis
The data from the last six months of the ICD device were analyzed. All the patients with evidence of at least one episode of appropriate ATP therapy were included. The first VT episode treated with ATP was evaluated to determine the success of ATP during the studied interval.
The ATP therapy was considered "successful" if the VT rhythm was terminated and did not recur for at least five seconds post-first ATP.
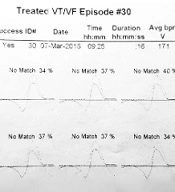
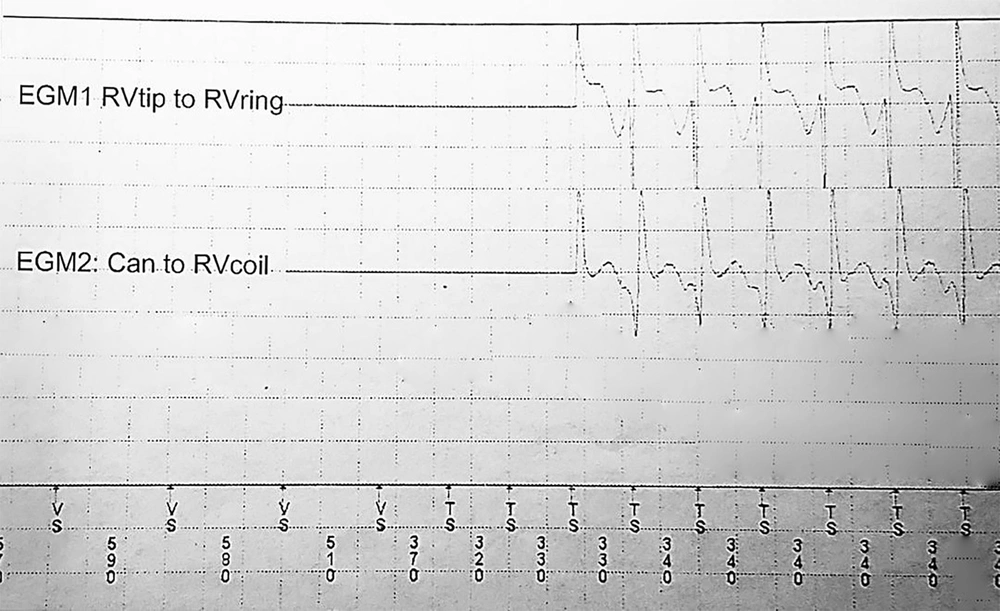
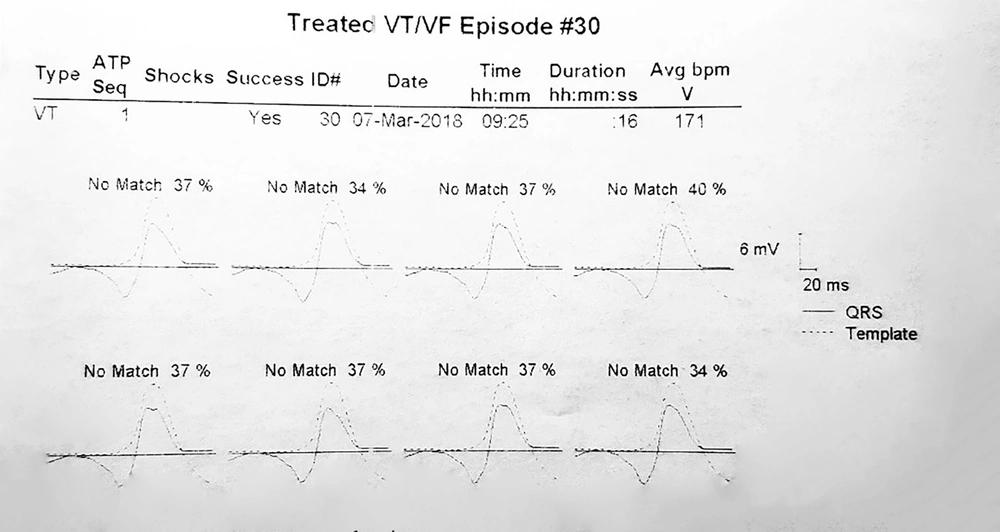
Pre-VT CL was defined as the mean "R-R intervals" in the last five sinus beats before the arrhythmia began. The ventricular far-field EGMs during VT and pre-VT episodes were morphologically classified as Q or non-Q based on the presence or absence of negative initial deflections with at least 40 milliseconds duration and a voltage exceeding 20% of the EGM amplitude (Figure 1). Furthermore, the VT CL, pre-VT CL, and VT time to ATP were extracted from the recorded EGMs. Using Medtronic devices, the average morphologic match between the last eight ventricular complexes during the VT rhythm prior to detection and the stored native QRS morphology was calculated and reported as matching percentages (Figure 2). Moreover, the far-field EGMs were divided into two groups based on the similarity of the initial deflection of the ventricular complex during the basal rhythm and VT episodes.
Example of calculating the matching percentage. The average morphologic match between the last eight ventricular complexes during VT rhythm prior to detection and the stored native QRS morphology was defined as a matching percentage. According to this tracing, the matching percentage averages 37, 34, 37, 40, 37, 37, 37, and 34%. So it is equal to 36.6%.
3.3. Data Acquisition
Demographic data, including age, sex, history of Coronary Artery Bypass Grafting (CABG) surgery and diabetes, type of device (ICD or CRT-D), antiarrhythmic drugs, left ventricular ejection fraction (LVEF), and type of cardiomyopathy were obtained from the patients' database.
3.4. Statistical Analysis
Continuous data were expressed as mean ± SD and categorical variables as percentages. The data were entered into SPSS software version 24.0. Chi-Square and Fisher's exact tests analyzed the association between ATP success and categorical variables, and its relationship with binomial variables was analyzed by independent t-test. P-values equal to or less than 0.05 were considered statistically significant.
3.5. Ethical Issues
The Scientific and Ethics Committee of Shahid Beheshti University of Medical Sciences approved the study (IR.SBMU.RETECH.REC.1396.556). All the patients provided informed consent, and no harm or additional costs were imposed on them.
4. Results
This retrospective study was conducted on 60 patients under the biannual analysis of ICD device implantations in Shahid Modarres hospital, Tehran, Iran. According to the EGMs recorded during the past six months, these patients had experienced at least one episode of ATP therapy. The mean age of the study population was 61 ± 12 years with a mean LVEF of about 26 ± 10%; 49 (81.7%) cases were male, and 56 (93.3%) were ICM (Table 1).
| Variables | All Patients (N = 60) | Successful ATP (N = 38) | Non-successful ATP (N = 22) | P-Value | OR (95% Confidence Interval) |
|---|---|---|---|---|---|
| Age (y), mean ± SD | 61 ± 12 | 62 ± 14 | 60 ± 6 | 0.57 | |
| Sex (male) | 49 (81.7) | 32 (84.2) | 17 (77.3) | 0.51 | 0.64 (0.17 - 2.40) |
| History of CABG | 22 (36.7) | 16 (42.1) | 6 (27.3) | 0.28 | 1.94 (0.62 - 6.05) |
| History of DM | 17 (28.3) | 11 (28.9) | 6 (27.3) | 1.00 | 1.09 (0.34 - 3.5) |
| Type of cardiomyopathy (Ischemic etiology) | 56 (93.3) | 34 (89.5) | 22 (100) | 0.29 | 1.65 (1.33 - 2.03) |
| LVEF | 26 ± 10 | 25 ± 10 | 28 ± 9 | 0.31 | |
| Device type (ICD) | 54 (90) | 33 (86.8) | 21 (95.5) | 0.40 | 3.18 (0.35 - 29.17) |
| Antiarrhythmic drug | |||||
| Beta-blocker | 41 (68.3) | 26 (68.4) | 15 (68.2) | 1.00 | 1.01 (0.33 - 3.12) |
| Amiodarone | 24 (40.0) | 15 (39.5) | 9 (40.9) | 1.00 | 0.94 (0.32 - 2.75) |
| Mexiletine | 9 (15.0) | 6 (15.8) | 3 (13.6) | 1.00 | 1.19 (0.27 - 5.31) |
| VT match percent | 33.37 ± 27.75 | 28.44 ± 28.63 | 27.63 ± 29.97 | 0.92 | |
| VT time intervals (ms) | |||||
| VT CL | 309 ± 46 | 313 ± 48 | 302 ± 44 | 0.38 | |
| Pre VT CL | 654 ± 156 | 661 ± 160 | 642 ± 154 | 0.65 | |
| ATP CL | 267 ± 39 | 271 ± 41 | 260 ± 34 | 0.29 | |
| VT time to ATP | 16 ± 20 | 15 ± 17 | 20 ± 25 | 0.35 | |
| VT morphology | 0.79 | 1.3 (0.45 - 3.76) | |||
| Q | 27 (45) | 18 (47) | 9 (41) | ||
| Non-Q | 33 (55) | 20 (53) | 13 (59) | ||
| Native QRS morphology | 0.29 | 1.6 (1.33 - 2.00) | |||
| Q | 3 (0.05) | 3 (0.8) | 0 (0) | ||
| Non-Q | 57 (83.3) | 35 (92.1) | 22 (100) | ||
| Native QRS-VT initial deflection match | 34 (56) | 21 (58) | 13 (59) | 0.79 | 0.85 (0.30 - 2.48) |
Abbreviations: OR, odds ratio; CABG, coronary arteries bypass graft; DM, diabetes mellitus; ICD, implantable cardiac defibrillator; LVEF, left ventricular ejection fraction; VT, ventricular tachycardia; CL, cycle length.
a Values are expressed as No. (%) unless otherwise indicated.
The ATP therapy was successful in 38 (63.3%) cases, and neither the demographic nor the clinical variables were significantly different between the two groups. The data analysis is discussed in detail below.
5. Discussion
Among the 60 EGM recordings of our study population, the ATP therapy was successful in about two-thirds of the patients, and none of the contributing factors affected the success rate.
Theoretically, reentry is an important mechanism in VT episodes, which can be interrupted by an ATP episode entering the circuit excitable gap. Therefore, shorter VT CLs (faster VT) with small excitable gaps seem to be more resistant to ATP therapies (18-24). However, this logic has not been observed in practice by all the current studies (25-27) since entering a stimulus into this gap can be affected by other variables, such as sympathetic activation, conduction time to the circuit, and the circuit (25).
In this study, the presence or absence of initial negative deflection in ventricular EGMs was significantly associated with ATP responsiveness neither during VT rhythm nor in the sinus rhythm prior to the initiation of arrhythmia. Previously, an initial Q wave during VT rhythm was reported to be associated with a higher ATP success rate by Jimenez-Candil et al. (26). Meanwhile, in a more recent study by Harrison et al., there was no association between QRS complex duration and morphology and ATP effectiveness rate (24). We also determined the pre-VT CL, ATP CL, VT to ATP delay, and the degree of similarity between QRS complexes during VT and native QRS morphology (matching percentages), which showed no association with the probability of successful ATP therapy. Moreover, we introduced a new variable, measuring the match between the initial deflection of ventricular complexes during the basal rhythm and VT episodes, which did not predict the possibility of ATP success rate.
This article revealed that demographic factors, including sex and age, were not predictors of effective ATP, and the history of DM or CABG was the same among the patients. Peters et al. suggested the female gender as a negative predictor of ATP success (28). Most studies have not confirmed the association between higher ATP failure rates and the history of ischemic cardiovascular events or background of ischemic versus non-ischemic cardiomyopathy (25, 26, 29).
It has already been suggested that impaired ventricular systolic function, irrespective of its etiology, is a predictor of ATP failure (26, 28), which has not been corroborated in all studies (21, 23-25).
Many patients with an ICD device also receive medical therapies to reduce appropriate ICD shocks, such as amiodarone and other antiarrhythmic and beta-blockers, which influence the electrical characteristics of cardiac myocytes and the conducting system. Beta-blockers are prescribed for all patients with reduced LVEF or a history of ischemia if the adverse effects are tolerated. They have been associated with greater ATP success rates in most previous studies (21, 23, 25, 26). In previous reports and ours, other antiarrhythmic drugs have shown non-significant efficacy in reducing ATP failures (21, 23, 26).
5.1. Conclusions
We did not find a strong predictor for successful ATP therapy, although expanding the study population may enhance the strength of non-significant associations observed in this population. It seems that the diversity of different processes beyond ventricular arrhythmias, even in a single patient, makes it too difficult to define the probability of ATP efficacy based on the patient's characteristics.
5.2. Study Limitations
The main limitation of this clinical investigation was the small number of patients with an ICD device who had experienced at least one episode of ATP therapy. Our study also lacked proper randomization and matching between the two groups of patients. Finally, we included only the patients carrying Medtronic devices to reduce the bias in defining EGM morphologies; such inclusion criteria can limit both the sample size and the applicability of the study.