1. Introduction
Since late 2019 to early 2020, the sudden outbreak of coronavirus disease 2019 (COVID-19) in Wuhan, China, was caused by the transmission of a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). The most common symptoms of COVID-19 are usually fever, dry cough, and fatigue; meanwhile, some symptoms, such as dyspnea and/or hypoxemia, that usually occur in severely and critically ill patients can rapidly progress to acute respiratory distress syndrome and coagulation dysfunction (2).
Many hypotheses have been identified for thrombosis occurrence among COVID-19 patients. Hypoxia can stimulate thrombosis by increasing blood viscosity and activating the hypoxia-inducible transcription factor-dependent signaling pathway. Multiple cellular and inflammatory responses activate the coagulation of inflammatory mediators. Moreover, respiratory failure and local hypoxia by venous stasis might lead to endothelial injury (3, 4). In addition, some situations, such as long-term bedridden, and likely receiving some treatments, such as glucocorticoids and intravenous immunoglobulin, and hormone therapy, increase the risk of venous thromboembolism (VTE) in severe COVID-19 (5, 6).
International guidelines suggest using standardized risk assessment models for evaluating prophylaxis in acutely ill patients. Therefore, with the increasing number of COVID-19 patients globally, most of whom are hospitalized, VTE prevention and diagnosis in COVID-19 patients is essential (4).
2. Objectives
The present study describes 39 cases of COVID-19 with VTE and reviews the clinical presentations and treatment strategies.
3. Methods
3.1. Patient Population
This case-series study was carried out at Masih Daneshvari hospital, a tertiary care hospital in Tehran, Iran. The COVID-19 cases were included based on polymerase chain reaction positive for SARS-CoV-2 or imaging compatible with the diagnosis of COVID-19, positive Doppler ultrasound of limbs for deep vein thrombosis (DVT), or chest computed tomography (CT) angiography positive for pulmonary thromboembolism (PTE). This study was approved by the Iran National Committee for Ethics in Biomedical Research (ethics code: IR.SBMU.NRITLD.REC.1399.112) and followed the ethical guidelines outlined in the 1975 Helsinki Declaration. Informed consent was obtained from all study participants before inclusion in the study.
Quantitative data were described by the mean and standard deviation (± SD). For qualitative data, the frequency and percentage were calculated.
4. Results
4.1. Demographic Data
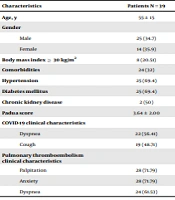
In this single-center case series, 39 COVID-19 patients diagnosed with PTE were reviewed between June and September 2021. Table 1 shows the baseline clinical characteristics of the patients. The mean age of the patients was 55 ± 15 years. Furthermore, 25 patients (64.10%) were male. The most frequent COVID-19 clinical presentations were dyspnea and cough in 22 (56.41%) and 19 (48.71%) patients, respectively. Additionally, the most frequent PTE clinical presentations were palpitation and anxiety in 28 (71.79%) and dyspnea in 24 (61.53%) patients.
| Characteristics | Patients (N = 39) |
|---|---|
| Age, y | 55 ± 15 |
| Gender | |
| Male | 25 (34.7) |
| Female | 14 (35.9) |
| Body mass index ≥ 30 kg/m2 | 8 (20.51) |
| Comorbidities | 24 (32) |
| Hypertension | 25 (69.4) |
| Diabetes mellitus | 25 (69.4) |
| Chronic kidney disease | 2 (50) |
| Padua score | 3.64 ± 2.00 |
| COVID-19 clinical characteristics | |
| Dyspnea | 22 (56.41) |
| Cough | 19 (48.71) |
| Pulmonary thromboembolism clinical characteristics | |
| Palpitation | 28 (71.79) |
| Anxiety | 28 (71.79) |
| Dyspnea | 24 (61.53) |
Demographic Characteristics a
A history of hypertension (n = 11; 28.20%) and diabetes mellitus (n = 8; 20.51%) were the most frequently reported. Moreover, a history of VTE was observed in 4 patients (10.25%). Furthermore, 11 patients (28.20%) were current tobacco smokers, all diagnosed with CT angiography. The color Doppler ultrasound showed DVT in 8 (20.51%) patients. In addition, 29 hospitalized COVID-19 patients (74.35%) presented VTE in a mean disease period of 7 days, and others presented with a mean time of 26 ± 5 days after their COVID-19.
Notably, D-dimer, high-sensitivity C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and ferritin as an acute phase reactant were higher than normal in all admitted patients. The mean measures of D-dimer, ESR, and CRP were 3680 ng/mL, 53 mm/hour, and 44 mg/L, respectively. Two-sided and one-sided ground-glass opacities on CT scans were observed in 23 (59%) and 16 (41%) patients, respectively. Eight cases (20.51%) had a body mass index higher than 30 kg/m2. Two cases (5.12%) were under ongoing hormonal treatment. Reduced mobility was observed in 23 patients (59%). The mean value of the Padua score was 3.64 ± 2.00.
4.2. Treatments and Outcomes
The therapeutic management involved an anticoagulant regimen, including low-molecular-weight heparins, rivaroxaban or enoxaparin, remdesivir, dexamethasone, tocilizumab, and supportive care based on the condition of patients (7, 8). Supplemental oxygen and ventilator were required in 29 (74.35%) and 2 (5.12%) patients, respectively. One patient died from PTE complications, and others improved without any complications.
5. Discussion
This study reviewed 39 COVID-19 patients with VTE as a critical complication in this disease, which showed high inflammatory factors and CT scan and CT angiography involvement in all patients. Multiple investigations demonstrated increased coagulation and cardiac biomarkers in patients with COVID-19, reflecting an inflammatory condition identified by coagulation activation, endothelial dysfunction, and mortality prediction (5, 9-13). Lodigiani et al. reported a notable frequency of venous and arterial thromboembolic events in hospitalized COVID-19 patients (~8%) in spite of using anticoagulant prophylaxis (14). The aforementioned study demonstrated the high risk of VTE development in patients admitted to the intensive care unit (ICU) (14). Nevertheless, the current case series showed that the majority of cases were diagnosed with VTE outside the ICU, including general and outpatient wards; only one case was admitted to the ICU.
In a cohort study conducted by Huang et al., in line with the present case-series study, most patients presented with fever, dry cough, dyspnea, and bilateral ground-glass opacities on chest CT scans (13).
As the 2019 novel coronavirus is an emerging virus, no antiviral treatment for coronavirus infection has been proven to be effective. Based on a systematic review conducted by Ansems et al., remdesivir had little or no effect on all-cause mortality up to the 28th day in hospitalized adults, and the effects of remdesivir on clinical improvement and worsening were uncertain (15). In this study, except for one case, both COVID-19 and VTE patients were improved with the routine treatment, including remdesivir and anticoagulant.
A study conducted by Zeng et al. on 274 COVID-19 patients demonstrated that severe and critical COVID-19 patients have a high risk of VTE and a higher Padua score, which was associated with poor prognosis (16). Nevertheless, in the current study, the low Padua score could not predict the VTE event.
Pasha et al., in a cohort study on 54,354 COVID-19 patients, showed a profoundly increased risk of VTE within the first week after positive testing for COVID-19 that returned to baseline levels after 6 weeks. They also observed VTE events almost exclusively in hospitalized patients, with the majority of them in the first days of hospitalization (17). The results of the aforementioned study are in line with the results of the present study that showed the most VTE events in the first week of COVID-19 patient admission and within about the first 4 weeks after discharge in the rest of the patients.
5.1. Conclusions
In conclusion, the present findings confirm that COVID-19 infection might be associated with increased rates of VTE. This study emphasizes thromboprophylaxis to avoid thrombotic events, especially for high-risk patients, the importance of these patients’ follow-up, and high clinical suspicion to the early detection of PTE and strengthens the importance of vaccination against COVID-19. Future clinical research would be beneficial in this regard.