1. Introduction
Ascending aortic pseudoaneurysm (AAP) is a surgical emergency requiring immediate medical and surgical attention and treatment. Unfortunately, AAP is undiagnosed in the majority of the cases, which makes it difficult to assess the epidemiology and incident of this life-threatening complication. Aortic pseudoaneurysm is defined as the accumulation of blood and connective tissue outside the aortic wall resulting from a contained aortic rupture. Previous cardiovascular surgeries, particularly aortic valve operations, are among the most important causes of such raptures (1).
2. Case Presentation
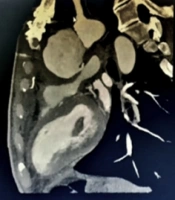
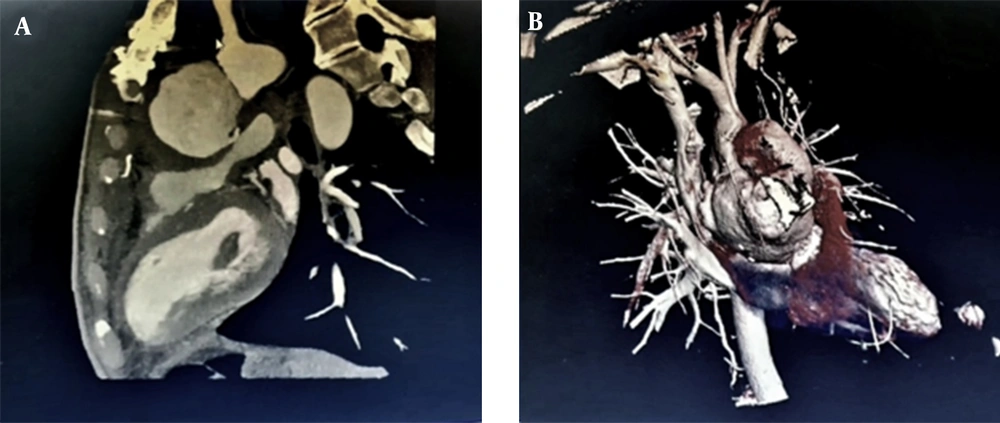
Our patient was a 47-year-old male admitted to the emergency ward with dyspnea, shortness of breath, and chronic localized chest pain. The patient had a history of median sternotomy due to aortic valve replacement because of aortic stenosis and was a known case of hypertension. After initial management, transthoracic echocardiography (TTE) revealed pseudo-aneurysmal blood accumulation within the aortotomy site. The diagnosis was then confirmed by a transesophageal echocardiogram (TEE). Computerized tomography (CT) and virtual rendering of CT scan also revealed an existing communication between ascending aorta and retrosternal space, suggesting ascending aorta rupture (Figure 1). Furthermore, the patient’s laboratory findings showed anemia, with hemoglobin of 6.7, which ameliorated to 10 before the surgery.
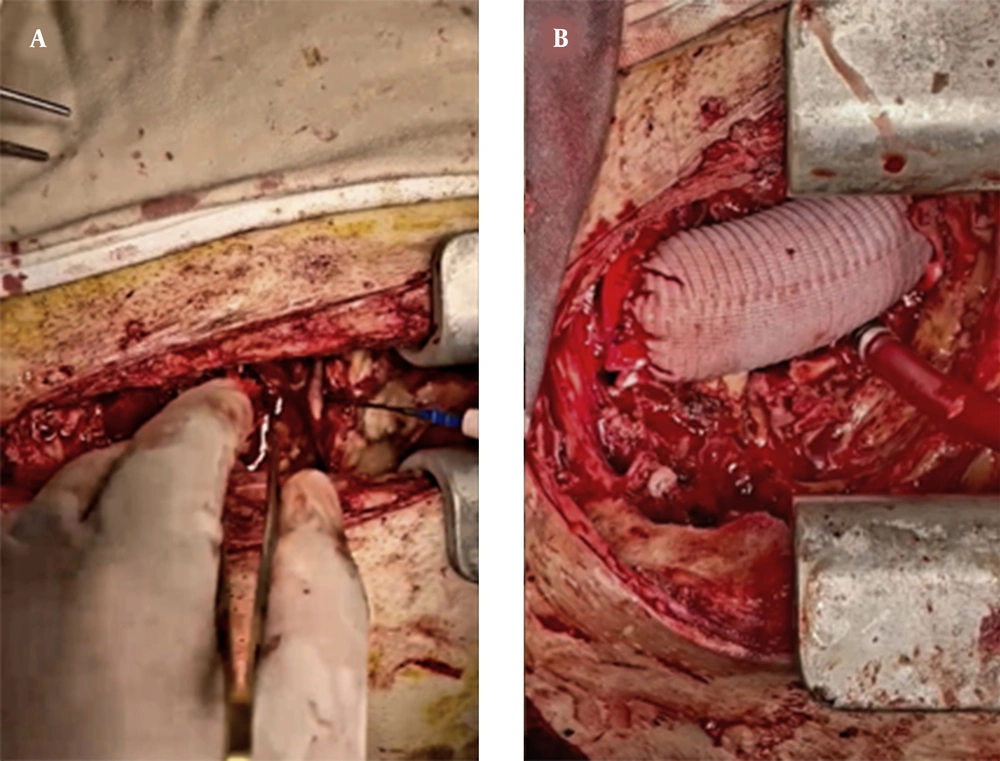
After the diagnosis was established, we decided to reoperate with the patient via right subclavian artery cannulation and simultaneous femoral artery cannulation. To impede the potential complications, we started cardiopulmonary bypass and cooled down the patient to 28°C and then up to 80°C. For the venous return, cannulation of the femoral vein was done. After the patient was cooled down and atrial fibrillation occurred, which was predictable due to a decrease in blood temperature, we performed a sternotomy. With entering through the sternum, the destruction of ascending aorta at the site of the previous aortotomy was obvious, and the aortic valve was exposed. Then, we approached the replacement of the complete root of ascending aorta. All of the necrotized materials were completely debrided out, and ascending aorta was replaced with a fibular graft (Figure 2).
3. Discussion
Aortic aneurysms are the leading cause of death among the general population (15th cause of death), especially in elderly patients aged more than 55 (10th cause of death). Unlike the overall decrease in the prevalence of abdominal aortic aneurysms, the prevalence of ascending aortic aneurysms (AAA) has increased over the past few years (2).
Several conditions could cause AAA based on the age of the patient. Ascending aortic aneurysms had been reported in several connective tissue disorders, such as Marfan, Loeys-Dietz, or Ehlers-Danlos syndromes, which are known as syndromic AAA. However, in patients aged more than 55, most cases are due to a degenerative event or are idiopathic or sporadic in several cases (3, 4).
Several risk factors have been suggested for AAA, including atherosclerosis, existing aneurysm, prior aortic dissection, chest trauma, aortitis (inflammatory or infectious), genetic predisposition, connective tissue disorder syndromes, familial AAA, aneurysm-osteoarthritis syndrome, bicuspid aortic valve, and prior history of aortic surgery or instrumentation. However, there are few reports of patients with preexisting aortic surgery who had secondary AAA (5). Although it is difficult for some physicians to consider a correlation between previous aortic surgery and AAA, in the absence of other risk factors and considering the age of the patient, assuming such a correlation seems rational. To find more evidence regarding the correlation between previous aortic surgery and AAA, we started to search for other similar investigations covering the same subject.
Schlosser et al. in 2008 studied the correlation between prior AAA and secondary AAA and evaluated the mortality and morbidity of these patients (5). They indicated a 2.2% AAA incidence in patients with prior AAA. This ratio was 2.5% in patients who developed a thoracoabdominal aortic aneurysm. This study did not report a significant correlation between prior aortic surgery (AAA in particular) and the development of AAA. Although there was no significant difference in the mortality rate of patients, a significant change in morbidity was reported in this study (5).
In 2003, Lombardi et al. investigated mortality and morbidity rates in patients with AAA with a prior history of aortic surgery (6). According to this study, 27% of patients reported a prior history of aortic surgery. Among them, infrarenal AAA was the most common prior procedure (20%). Besides, 7% of patients needed reoperation after failed AAA and reported a high incidence of Marfan syndrome (6).
Although suffering from AAA after aortic valve replacement is extremely rare, Derkac et al. in 1974 reported a 51-year-old male patient with dissecting aortic aneurysm a year after aortic valve replacement surgery (7).
In a retrograde perspective study by Atik et al., pseudoaneurysms of the aorta, in general, were studied (8). They reported that from 60 patients who underwent aortic pseudoaneurysm surgery, 59 (83%) patients had prior cardiovascular surgery. Of them, 22 (37%) patients had valvular graft operations, suggesting a correlation between cardiovascular surgery (even valvular operation) and aortic pseudoaneurysm (8).
While AAP is known to be a life-threatening emergency, it is a manageable pathology that can be treated to save patients’ lives through suitable actions. Recently, Fukunaga and Koyama studied the surgical complications and outcomes of such patients with a prior history of cardiac surgery and revealed no aortic death in 20 patients involved in the study (9).
3.1. Conclusions
Based on our search, only a few cases have been reported concerning the correlation between previous aortic surgery and AAP. However, a prior aortic valvular surgery, or in a more general concept, previous aortic surgery has been proven to be of importance in developing AAP. Our team suggested that in patients with similar profiles, previous aortic surgery should be taken into account until a more thorough diagnosis and management can take place.