1. Introduction
Eosinophilic myocarditis (EM) is a rare cause of acute myocarditis. Initial presentations vary between non-specific symptoms to acute heart failure and cardiogenic shock and can even be fatal. The chronic process might lead to fibrosis and restrictive cardiomyopathy (1), and the course might differ in different patients. Initial diagnosis is based on the clinical, laboratory, and imaging manifestations of myocarditis, including electrocardiographic changes, elevated cardiac biomarkers, and evidence of cardiac functional impairment and inflammation in echocardiography, magnetic resonance imaging (MRI), and endomyocardial biopsy (2). Definite diagnosis needs proof of the existence of eosinophilic infiltration or its consequences, such as myocardial fibrosis or necrosis in the endomyocardium (2). Herein, we present a case of EM; although it was not a typical case accompanied by all the criteria, some pathologic and laboratory clues led to appropriate diagnosis and treatment.
2. Case Presentation
A 53-year-old female with a history of asthma since many years ago and coronavirus disease 2019 (COVID-19) infection two weeks ago was admitted with dyspnea, pleuritic chest pain, and scattered neuropathic pains since one week ago. The patient also had complaints of blurred vision since the time of the COVID-19 infection. Moreover, the patient mentioned a history of vitrectomy due to trauma. A family history of rheumatoid arthritis was positive in her brother.
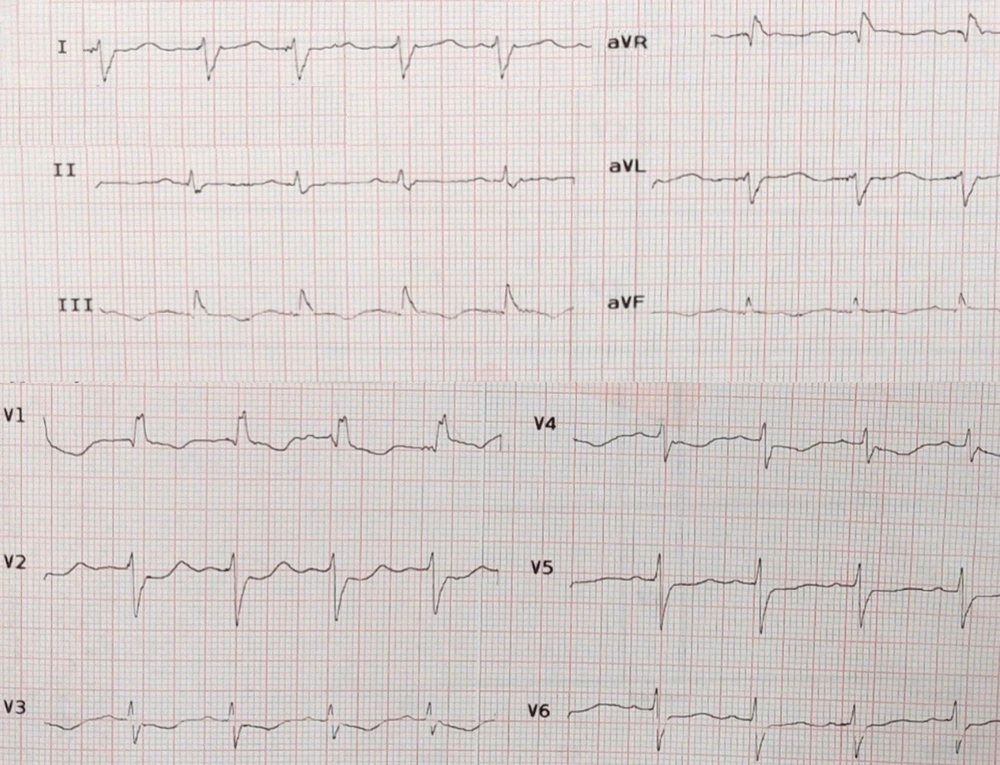
On admission, blood pressure, pulse rate, oral temperature, and oxygen saturation were reported as 100/80 mmHg, 85 beats/minute, 36.7°C, and 94%, respectively. Electrocardiogram revealed sinus rhythm with the right bundle branch block (Figure 1). She underwent transthoracic echocardiography (TTE) one week before admission, which reported a normal left ventricular size with left ventricular ejection fraction (LVEF) of about 50 - 55%. In our center, Modarres Hospital, TTE was repeated and demonstrated LVEF 20 - 25% with global hypokinesia, normal right ventricular size and function, and unremarkable valvular disease. A chest X-ray was unremarkable except for moderate size left-sided pleural effusion, which was confirmed by a chest computed tomography scan. The passive collapse of the lingula and left lower lobe was also reported.
Laboratory tests revealed a troponin I level of 1.44 ng/mL (normal < 0.03 ng/mL), urea level of 42 mg/dL, creatinine level of 0.8 mg/dL, aspartate aminotransferase level of 46 U/L, alanine aminotransferase level of 48 U/L, white blood cell count of 14.8 × 103/microliter, neutrophil level of 72%, a hemoglobin level of 11.4 g/dL, thyroid stimulating hormone level of 1.3 mIU/mL, and N-terminal pro-B-type natriuretic peptide level of 13100 pg/mL (normal level < 120 pg/mL). On the third day of admission, we asked for an eosinophil count, which was transiently 38% only in one sample.
Due to the rapid decrease in LVEF, dyspnea, and chest pain, acute coronary syndrome and acute myocarditis were two main differential diagnoses. In order to evaluate the coronary arteries, coronary angiography was performed, which revealed normal epicardial coronary arteries. Based on the initial diagnosis of acute myocarditis, the patient received two doses of intravenous methylprednisolone (500 and 250 mg), which was continued by oral prednisolone. Eosinophil count became normal after receiving prednisolone, and the troponin level started to decrease. The LVEF improved by 40%, and symptoms of heart failure were relieved after diuretic therapy and standard heart failure treatment.
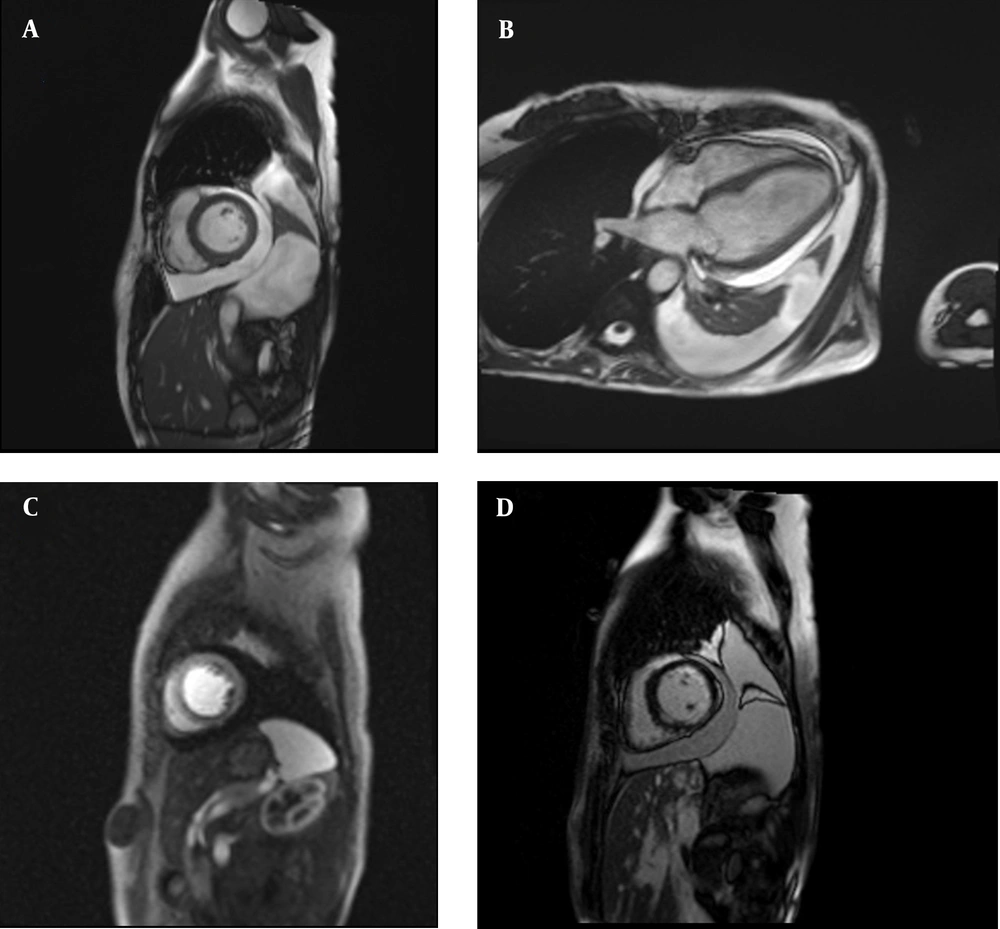
The evaluation of the cause of myocarditis was continued, and an endomyocardial biopsy was performed, which reported evidence of inflammatory cell infiltration and necrosis but not specific to a special cause. Stool examination was negative for parasitic infection and mutations for JAK2 and PDGFR (to evaluate eosinophilia). Rheumatologic tests were also negative. Furthermore, the ophthalmologic study was unremarkable. Finally, the patient was diagnosed with EM based on the presence of subendocardial edema and late gadolinium enhancement in cardiac MRI (Figure 2). Oral prednisolone, 15 mg per day, was continued after intravenous pulse concomitant with anti-heart failure medications (i.e., spironolactone, carvedilol, empagliflozin, and enalapril). During the follow-up period, LVEF improved to 50% after one month, and the case remained asymptomatic.
A and B, Short axis and four-chamber view of cine steady-state free precession sequence showing large pericardial effusion and pleural effusion; C, short axis dynamic perfusion sequence showing subendocardial perfusion deficit; and D, short axis late gadolinium enhancement sequence showing subendocardial hyperenhancement
3. Discussion
As inflammatory myocarditis, EM affects both men and women, mostly around 40 years (3). Irrespective of low sensitivity (about 50%), an endomyocardial biopsy is still known as the gold-standard method for the diagnosis (4-6). The presence of biventricular apical clots is reported in similar cases that might be diagnosed by TTE or cardiac MRI (7).
There are some pieces of evidence in favor of a genetic basis for EM (5). Allergies, drug hypersensitivities, viral or parasitic infections, and hematologic causes, such as myelodysplasia, systemic vasculitis, and some tumors, have been the known etiologies of eosinophilia which can lead to myocardial involvement (5, 8, 9). Moreover, the presentations can be similar to other types of myocarditis.
Although echocardiography is usually the initial non-invasive imaging, cardiac MRI can reveal capillary leakage, edema, local inflammation, and fibrosis in myocarditis (5). The treatment strategy is based on appropriate immunosuppression therapy and treatment of heart failure symptoms (5).
In contrast to some of the reported cases (8), the present case did not have any fever or malaise on admission, and peripheral eosinophilia was only reported in a complete blood count, which made the diagnosis very difficult. An important finding in her past medical history was asthma which was occasionally treated with some inhalers. The evaluation of related mutations and rheumatologic tests were negative, which is indicative of idiopathic hypereosinophilic syndrome. Cardiac MRI was the main diagnostic tool in our case due to the inconclusive results of the endomyocardial biopsy. As previously mentioned, the low sensitivity of endomyocardial biopsy might cause the diagnosis difficult. Additionally, previous reports have emphasized the role of cardiac MRI in addition to endomyocardial biopsy (3, 4).
The appropriate response to steroid therapy is an important feature of EM which is also mentioned in the present case. Nevertheless, there is not enough evidence about the acceptable duration of treatment (8). The best treatment strategy should be considered based on the underlying etiology.
There are some reports about the presence of EM after COVID-19 infection. The present case had a history of COVID-19 infection two weeks prior to admission.
.jpg)