1. Introduction
Symptomatic chronic mesenteric ischemia (CMI) is a rare condition that usually occurs due to mesenteric artery stenosis (MAS) with a common incidence (1, 2). The prevalence of symptomatic CMI is less than 2%, while the prevalence of MAS is 6%, 14%, and 18%-67% at ages 40, 60, and more than 75 years old, respectively (3). CMI is more common in women than men (2:1) (3, 4). The mesenteric arterial circulation is performed by the celiac artery (CA), superior mesenteric artery (SMA), and inferior mesenteric artery (IMA), which originate from the aorta at the level of the crux of the diaphragm, 2 cm below the CA, and splenic flexure, respectively. The normal diameter of CA is 6 mm, SMA 7 mm, and IMA 2 mm (5).
2. Case Presentation
A 60-year-old woman with diabetes was hospitalized with prolonged abdominal pain, primarily localized in the epigastric area. The abdominal pain usually began with eating and lasted for hours. The analgesics were used to subside the pain. In the physical examination, a diffuse mild abdominal tenderness disproportionate to the pain with no rebound or guarding was detected. An abdominal bruit was heard by auscultation. An abdominal ultrasonography was performed, and a dilated common bile duct (CBD) and a gallstone were diagnosed. The patient was a candidate for endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasonography (EUS) because dilated CBD was reported by ultrasonography. After ERCP, the patient was discharged without any pain. A laparoscopic cholecystectomy was performed 2 days before her discharge.
She was readmitted again to the emergency ward for abdominal pain, especially in the epigastric area. Her pain started from eating. She feared eating and had lost 5 - 6 kg weight during the last 6 months. An imaging reevaluation was performed by computed tomography angiography (CTA), which showed total occlusion of SMA with a long and calcified lesion, decreased blood flow in IMA, and normal blood flow in the truck of CA.
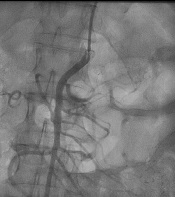
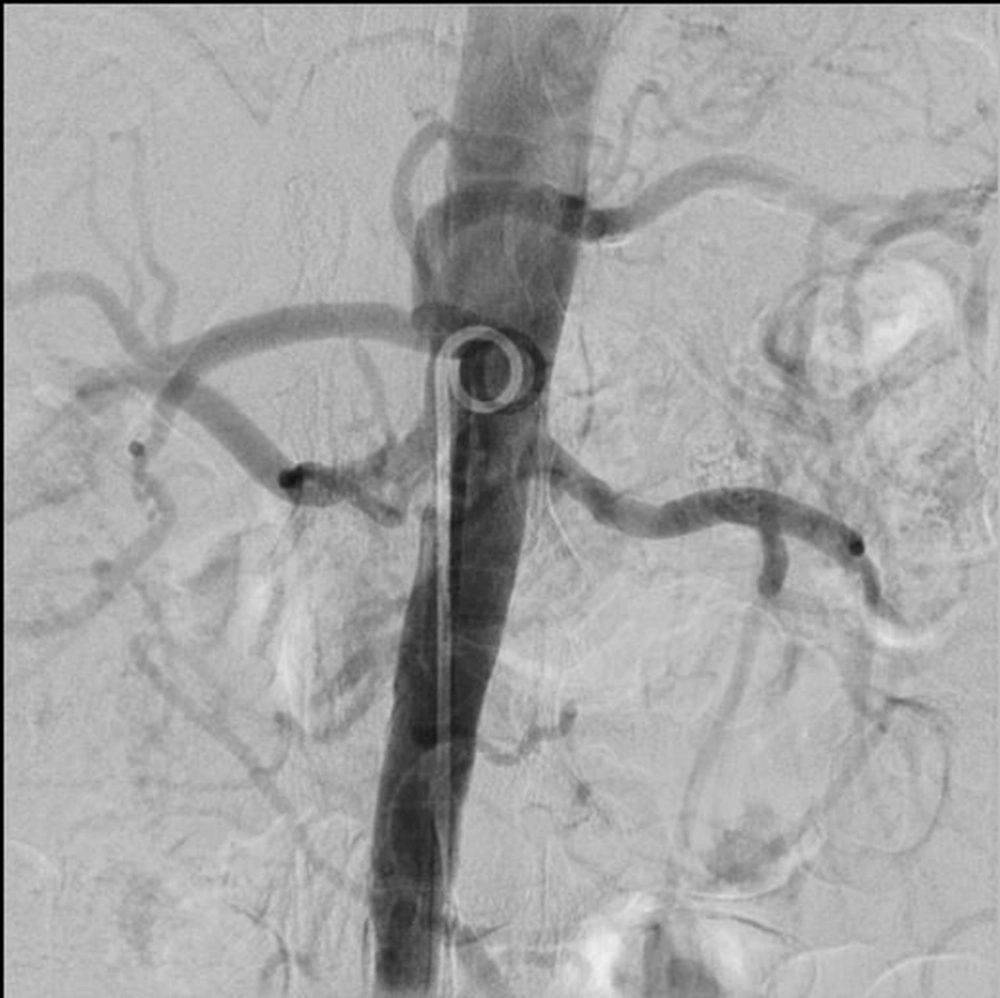
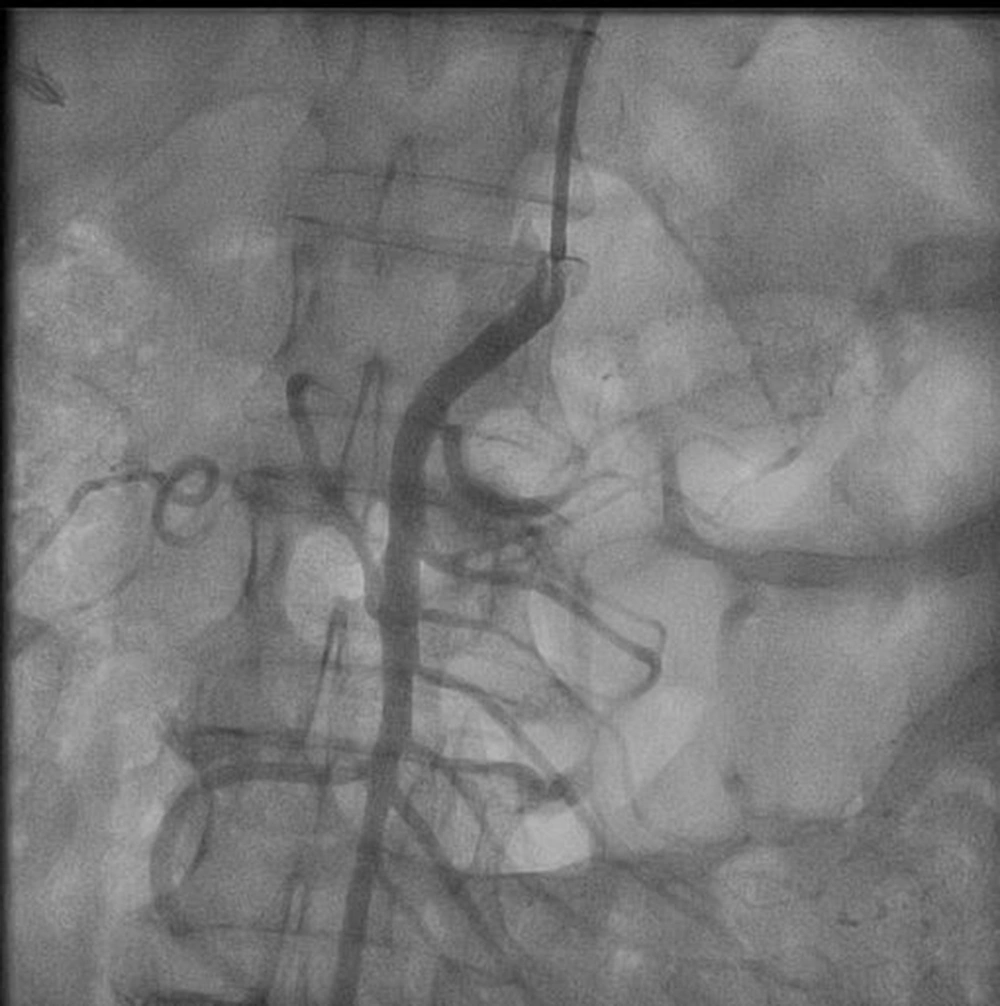
The patient was a candidate for an endovascular or open surgical procedure. Considering her comorbidities and age, she was a candidate for an endovascular treatment approach. At the first attempt, cannulation of total SMA cut off via the right femoral artery approach was unsuccessful. However, after changing the approach to the brachial access and multipurpose catheter, the SMA origin was found easily, helping calcification in fluoroscopy. Next, a 0.014-inch pilot wire showed partial thrombosis, and stent 4-33 Xience was deployed with an excellent final result (Figures 1 and 2).
The abdominal pain disappeared, and all the analgesic agents were stopped immediately after percutaneous mesenteric artery stenting (PMAS). After three months of follow-up, the patient was in good condition without recurrent abdominal angina.
3. Discussion
The common causes of MAS and particularly CMI are the same as the atherosclerotic process that leads to narrowing the surface of arteries, such as hypertension, hyperlipidemia, overweight, metabolic syndrome, and smoking. Other causes of MAS include vasculitis, fibromuscular dysplasia, Takayasu's arteritis, Buerger's disease, compression by the median arcuate ligament, referred to as median arcuate ligament syndrome, radiation, and cocaine abuse (2, 3, 6). As represented in the literature (2, 3, 7), our patient had diabetes as a risk factor for MAS. Unlike peripheral artery disease, coronary artery disease, and cerebrovascular diseases, the incidence of CMI is higher in women (2), as represented in our case.
The postprandial pain that usually starts by finishing a meal and lasts 30 minutes to 2 hours is the common presentation of CMI that occurs in 90% of the patients (2, 3). A fear of eating leads to taking smaller and fat-free meals, which causes weight loss and vitamin insufficiency in 85 - 100% of the patients (3, 8-10). Unexplained diarrhea has been reported in 7 - 35% of the patients as a sign of end-stage CMI. Abdominal pain following meals, weight loss, and diarrhea are common in other gastrointestinal disorders, including peptic ulcer disease, chronic pancreatitis, inflammatory bowel disease, functional dyspepsia, and irritable bowel syndrome (2, 7).
A typical CMI is usually represented in patients with multi-vessel stenosis (at least two vessels). Single-vessel stenosis, particularly SAM stenosis, is usually asymptomatic because of collateral arteries' blood flow (2, 3, 11). The diagnosis of CMI is confirmed by a medical history of insufficient blood flow of mesenteric arteries (at least two vessels), the stenosis of mesenteric arteries of more than 70 - 75%, and an actual affirmation of stenosis (8, 12).
The CTA, with a sensitivity of 95% and specificity of 100%, is the best imaging modality for diagnosing CMI in moderately highly suspicious patients (13). Magnetic resonance angiography (MRA) may be performed in a patient with contrast allergy or renal insufficiency (2). The duplex ultrasonography (DUS) can be used with a sensitivity of 70 - 100% for 70% stenosis of CA, 72 - 100% for 70% stenosis of SMA, and specificity of 77 - 90% and 84 - 98% for CA and SMA, respectively (13). An operator dependent on DUS is a weakness of this modality.
Two treatment approaches for revascularizing the mesenteric arteries are indicated in patients with occlusive CMI to improve the symptoms. The open surgical revascularization approach has been the most common therapeutic approach for the last two decades. The most common location of mesenteric arteries stenosis is the ostium of the arteries. The first endovascular approach was used in the 1980s. The endovascular approach has been performed through transbrachial, transfemoral, and transradial (2, 3). Transradial PMAS is safer than the transbrachial approach.
An open surgical approach is performed when the endovascular revascularization fails or cannot technically be performed. There are some contraindications for the open surgical approach, such as a lower technical success rate and/or increased procedural complications that may occur more in tortuous aorta-iliac arteries, long-segment occlusion, small-diameter distal vessels, and heavily calcified stenosis (3, 7, 13-15). Altogether, the success rate of the endovascular approach and open surgical revascularization are 85 - 100% and 97 - 100%, respectively. In comparison, the endovascular revascularization complications are less than open surgical revascularization at 13 - 40% versus 0 - 31% (3). As reported, 28 - 36% of restenosis may occur in patients treated by the PAMS approach, which is less common than in patients treated by the surgical approach (0 - 25%). One of the severe complications of endovascular revascularization is distal embolism which may occur in severe mesenteric calcification, occlusions, longer lesions, and small vessel diameter (2, 3). In-hospital complications are significantly higher in the surgical revascularization approach (2). A routine follow-up by DUS is recommended 1, 3, 6, and 12 months later (6, 16). As represented in our study, we followed up with our patient after 3 months, and there were no complications or pain.