1. Background
Congenital heart disease is a significant cause of heart failure and death due to cardiovascular events in adults younger than 50 years of age. Ebstein’s anomaly (EA) is a rare form of right ventricular cardiomyopathy and tricuspid valve dysplasia and accounts for less than 1% of patients with congenital heart diseases (1). This anomaly is characterized by variable levels of tricuspid valve (TV) septal and posterior leaflet displacement due to the abnormal delamination of the underlying right ventricular (RV) myocardium, resulting in severe tricuspid regurgitation (TR) and abnormal atrialized portion of RV, culminating in right-sided heart failure. Variable degrees of leaflet displacement are accountable for numerous anatomic subtypes of EA (2, 3), and clinical presentation ranges from intrauterine or shortly after birth mortality to minimal symptoms in early adulthood. Due to the wide spectrum of clinical and anatomic presentations, finding a uniform management strategy for EA is often challenging (2, 3).
Most patients with EA require surgery sometime in their lifetime. Historically, there was a tendency to postpone the surgery considering the poor durability of repair techniques and the inherent complications of inserting a prosthetic valve in the right-sided cardiac positions. Hunter and Lillehei first reported a surgical technique to repair EA in 1958 (4). Since then, numerous techniques have been proposed to eliminate TR and restore RV geometry (5-11). Cone repair has shown the most promising results in recent years and is currently the operation of choice in our institution. During cone repair, the severity of TR is reduced by constructing a funnel-like valve similar to the physiologic TV (6, 7, 12-14).
2. Objectives
In the current study, we sought to evaluate the echocardiographic and clinical outcomes of cone repair in EA patients who underwent surgery in our institution.
3. Methods
The current investigation reviewed the records of 56 consecutive EA patients presenting to our referral cardiovascular center between May 2015 and June 2021. Patients with previous palliative surgery or no surgery, individuals with remarkable coronary artery disease, and those who lacked post-op and follow-up echocardiography data were excluded. Ultimately, 35 patients who fulfilled the eligibility criteria were evaluated. All patients underwent follow-up echocardiographic examination at least one year after the operation. Demographic data, including age, sex, and operation details, were extracted from patient records. The primary endpoints of the study were baseline TV anatomy, residual TR severity, and right and left ventricular sizes and function.
Transthoracic echocardiographic examination was performed through echocardiography by adult congenital heart disease fellowship specialists. The Philips Affiniti 70 ultrasound and GE Vivid S60 devices were used to obtain echocardiographic images. Color Doppler echocardiography was used to evaluate tricuspid valve regurgitation. Valvular regurgitation severity was defined based on the guidelines of the American Society of Echocardiography (15). The diameter of the left ventricle (LV) was determined on the parasternal long axis view, and the line used for measurement was oriented perpendicular to the long axis of the left ventricle at the level of the mitral valve leaflet tips. Left ventricle ejection fraction was estimated in the apical four-chamber view using the Simpson method. Right ventricular size was also determined in the apical four-chamber view; RV internal diameter was regarded as the width of the middle one-third of the RV (16). The fractional area change (FAC) was measured by tracing the RV endocardium in an apical four-chamber focused view for RV during diastole and systole (17, 18). The patients were divided into two groups (those undergoing tricuspid valve replacement and those subjected to tricuspid valve repair) to compare the post-op results.
The decision for the patients to undergo TV replacement or cone repair was based on the surgeon’s discretion and whether they had expertise in performing cone repair or not. Indications for surgery included desaturation and cyanosis, symptomatic heart failure, atrial or ventricular arrhythmias refractory to other therapies, declining left ventricular systolic function, and progressive RV dilatation and dysfunction. Indications for undergoing corrective surgery were the same in the replacement and repair groups.
The operative strategy consisted of the following steps based on preoperative imaging and clinical findings: (1) Cone reconstruction of the TV or valve replacement (biologic or mechanical); (2) correction of any associated anomalies, such as right ventricular outflow obstruction, pulmonary stenosis, and patent ductus arteriosus; (3) complete or partial closure of ASD or PFO when the right ventricular function was insufficient; (4) plication of the atrialized part of the right ventricle; and (5) bidirectional cavopulmonary shunt when there was severe RV dysfunction, or the functional RV size was small.
Surgical details during cone repair have been reported previously (6, 7). The operation consisted of the circumferential delamination or separation of the leaflet from the underlying right ventricle. The free leaflets were then joined side to side to form a circumferential cone, and the cone of the leaflet tissue was then attached to the anatomic TV annulus. Leaflet augmentation, autologous neochordae, and ring annuloplasty were utilized at the surgeon’s discretion during the operation.
The Institutional Ethics Committee approved the study protocol, and the institutional review board waived the requirement for obtaining informed consent because of the retrospective nature of the study.
3.1. Statistical Analysis
Numerical variables were presented as mean ± standard deviation (SD), and categorical nominal or ordinal variables were expressed as percentages. The assumption of normality was assessed by the one-sample Kolmogorov-Smirnov test. Differences between the study groups were evaluated using Mann-Whitney U test. When the significance level (P < 0.05) was exceeded, the Bonferroni post-hoc test was utilized to discern the difference between the groups and to rectify multiple comparisons. One-way analysis of covariance (ANCOVA) was performed to adjust the baseline differences between the study groups (α = 0.05). The comparisons were made using nonparametric tests, including the Mann-Whitney U test. For data analysis, IBM® SPSS® Statistics version 26 was used, and P values less than 0.05 were considered statistically significant.
4. Results
From May 2015 to June 2021, 35 consecutive patients underwent corrective surgeries for EA. Twenty-one (60%) patients underwent cone repair, and 14 (40%) patients were managed with TV replacement. The mean age of the patients at the time of operation was 29.49 ± 9.13 years (Table 1). Sixteen (45.7%) patients were male, of whom 9 (64.3%) were in the TV replacement group, and 7 (33.3%) were in the cone repair group. Among female patients, 5 (35.7%) underwent TV replacement, and 14 (66.7%) cone repair. The mean ages of the patients in the TV replacement and cone repair groups were 30.1 ± 8 and 28.3 ± 9.2 years, respectively. There were no significant differences in the demographic and clinical characteristics of the patients in the two groups. The decision for the patients to undergo either TV replacement or cone repair was based on the surgeon’s discretion and whether he/she had expertise in performing cone repair or not.
Abbreviations: RV, right ventricle; FAC, fractional area change; LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
a Values are expressed as mean ± SD.
b P values less than 0.05 were considered statistically significant.
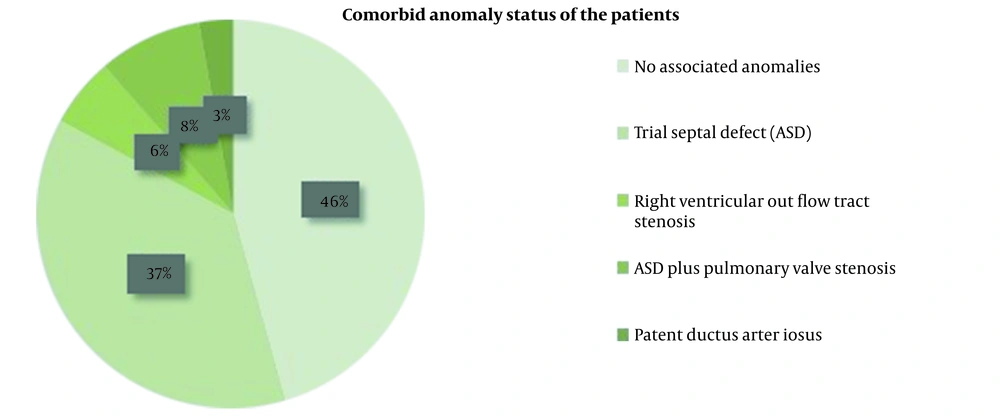
Indications for surgery included desaturation and cyanosis, symptomatic heart failure, atrial or ventricular arrhythmias refractory to other therapies, deteriorating left ventricular systolic function, and progressive RV dilatation and dysfunction. Indications for performing corrective surgery were the same in the replacement and repair groups. Sixteen (45.7%) patients had no associated anomalies; 13 (37.1%) patients had atrial septal defects (ASD); 2 (5.7%) patients suffered from right ventricular outflow tract stenosis; 3 (8.6%) patients had ASD and pulmonary valve stenosis, and one patient (2.9%) had patent ductus arteriosus (PDA) (Figure 1).
Among the participants, seven patients (20%) required reoperation during the follow-up. Three patients (8.6%) in the cone repair group, who had previous TV repairs, suffered from severe residual tricuspid regurgitation and underwent redo-surgery. Four patients in the valve replacement group needed reoperation, two of whom underwent biologic TVR (5.7%), and one patient was managed with mechanical TVR (2.9%) due to degenerative changes and destruction of previous biologic TV. Finally, one patient underwent mechanical TVR (2.9%) due to the malfunction of the previous mechanical TV.
In the cone repair group, 12 patients (34.3%) had mild residual TR; six patients (17.1%) had moderate residual TR, and three patients (8.6%) had severe residual TR. In 14 patients (40%) with TVR, no paravalvular and transvalvular regurgitation was reported.
Mean anatomic RV size increased post-surgery, but this increase was not statistically significant irrespective of the type of operation (P = 0.38 in both). After the operation, the absolute value of RV-FAC significantly increased (P < 0.001).
Changes in the mean left ventricular end-systolic diameter and the mean left ventricular end-diastolic diameter did not reach the statistical significance level (P = 0.302 and P = 0.039, respectively). Changes in the absolute value of the average 2D-LV ejection fraction (2-D LVEF) before and after the operation were statistically significant in both groups (P < 0.001, Table 2).
| Variables | TV Replacement (n = 14) | Cone Repair (n = 21) | Between-Group Comparison | ||||
|---|---|---|---|---|---|---|---|
| Before Operation | After Operation | P-Value | Before Operation | After Operation | P-Value | P-Value | |
| RV size (cm) | 4.18 ± 0.43 | 4.50 ± 0.33 | 0.020 b | 4.14 ± 0.37 | 4.23 ± 0.43 | 0.48 | 0.063 |
| FAC (%) | 33.00 ± 7.83 | 40.07 ± 8.50 | < 0.001 b | 38.05 ± 4.95 | 45.48 ± 5.97 | < 0.001 b | 0.355 |
| LVESD (cm) | 2.00 ± 0.21 | 2.05 ± 0.16 | 0.548 | 2.00 ± 0.21 | 2.01 ± 0.16 | 0.827 | 0.475 |
| LVEDD (cm) | 4.11 ± 0.35 | 4.14 ± 0.27 | 0.818 | 4.12 ± 0.41 | 3.94 ± 0.32 | 0.029 b | 0.050 b |
| 2D-LVEF (%) | 38.50 ± 6.35 | 45.36 ± 6.34 | < 0.001 b | 42.86 ± 7.67 | 49.05 ± 6.25 | < 0.001 b | 0.472 |
Abbreviations: TV, tricuspid valve; RV, right ventricle; FAC, fractional area change; LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
a Values are expressed as mean ± SD.
b There was no significant correlation between the degree of apical displacement of septal tricuspid valve leaflets and postoperative RV size and FAC.
5. Discussion
The goal of surgery in EA is to restore the entire anatomic RV to a functional ventricle in order to achieve a better effective stroke volume and RV function. In 1988, Carpentier et al. defined a new repair method that comprised the longitudinal plication of the RV, returning the TV to the anatomic level and reinforcing it with a prosthetic ring (8). Danielson et al. subsequently introduced transverse plication of the atrialized RV, right reduction atrioplasty, and posterior tricuspid annuloplasty (9). All these techniques delivered a considerable rate of residual TR, so TV replacement was necessary on many occasions. In 2004, Silva et al. described a new and encouraging surgical technique, namely cone repair, that used some principles of the Carpentier et al.’s method (8, 10). The new approach included moving the tricuspid leaflets to the anatomic tricuspid annulus level, accompanied by the longitudinal plication of the atrialized RV, resembling the normal TV anatomy (10). In addition to improving TR, cone reconstruction better restore RV function and geometry. Long-term outcomes, as reported by da Silva and da Silva, were favorable, with a low mortality rate and satisfactory TV performance (11).
In our center, the cone reconstruction technique has been used increasingly in recent years. In this study, we found that cone repair significantly reduced TR severity post-surgery and led to a significant increase in RV-FAC and LVEF. These results were similar to that reported by Silva et al. (19) and Nihat and Kara (20), who reported good RV function and a low incidence of recurrent severe TR in the long-term follow-up. Some studies evaluating the outcomes of cone operation in EA patients by cardiac magnetic resonance imaging have demonstrated a marked reduction in TR, RV end-diastolic volume index (EDVi), RV end-systolic volume index (ESVi), and RV stroke volume index (SVi), however, RVEF remained unchanged (12, 21). In 2018, Perdreau et al. reported that RV-FAC was significantly reduced post-operation (22). These studies evaluated the patients early after the operation (less than seven months post-op), whereas we followed patients up for at least one year after the operation, encompassing the necessary time required for RV recovery and positive remodeling. In accordance with our findings, Ibrahim et al., using serial echocardiographic assessment of RV function, showed improved RV systolic function after 24 to 36 months of surgery and hypothesized that the eradication of TR and improved forward flow in the pulmonary artery could present as enhanced RV myocardial performance several years after the repair (23). It has been noted that tricuspid regurgitation is remarkably reduced after cone reconstruction; however, RV function typically takes longer to improve (24).
The present research revealed a statistically significant increase in LVEF and a decline in LVEDD; however, LVESD remained unchanged. Ibrahim et al. showed that global LV function remained unchanged and within normal limits (23). In the recent study, systolic LV eccentricity index and LV shortening function did not significantly change as well post-surgery. However, cardiovascular magnetic resonance analysis showed that cone reconstruction improved LV filling due to the enhanced forward blood flow following a significant reduction in TR severity (23). Moreover, Rotar and Kron found no changes in the left-sided ejection fraction, global circumferential, and longitudinal strains (24). However, basal septal circumferential strain improved with a median of 2.8 years after cone reconstruction. These researchers attributed the reduction in RV end-diastolic volume to the increase in LV end-diastolic volume mediated via ventricular-ventricular interactions (12, 24).
5.1. Limitations
Studies on EA are generally limited by the small sample size as this anomaly is a rare condition. Analysis of pre- and postoperative data from the same individuals helped us perform comparative analysis on a wide range of patient demographics. Almost all readily applicable measures of RV and LV systolic function are load-dependent and can be affected by alterations in preload and afterload volumes following surgery. Improved TR severity seems to be a more reliable marker for evaluating surgical outcomes.
5.2. Conclusions
Durable TV repair is an important issue in young patients with EA. Cone repair is a safe and effective method to resolve tricuspid regurgitation and restore near-anatomic RV geometry. Follow-up echocardiography in our study showed a low incidence of residual severe tricuspid regurgitation and improved biventricular functional parameters after the corrective surgery. The learning curve for cone reconstruction is steep, with a difficult initial learning process, but it is essential for surgeons working in institutions dealing with congenital heart disease patients to master the skill.