1. Context
As we know, atrial fibrillation (AF) is the most prevalent sustained cardiac arrhythmia (1). It is an irregular and occasionally rapid arrhythmia originating from multiple ectopic foci in the atria (2). AF is known to be an important risk factor for dementia, ischemic stroke, heart failure, and decreased quality of life (3-7). The estimated risk for the incidence of this arrhythmia is 25% in people aged over 40 years (8, 9). AF affects more than 33 million people worldwide, and its overall incidence is 5 million new cases annually (10). Therefore, AF is an important public health concern. Some risk factors, including diabetes mellitus, hypertension, smoking, increased body weight, alcohol consumption, and stress, are proposed to be associated with AF (11).
Mitochondria are cellular organelles with a double-layer membrane that convert various forms of molecules into adenosine triphosphate (ATP). They do this process via ATPase enzyme and in the presence of electrons that have high energy in the internal mitochondrial membrane in a process named oxidative phosphorylation (12, 13). Mitochondria have a double-stranded, small, circular, independent DNA (known as mtDNA) that encodes 2 ribosomal RNAs (rRNAs), 22 transfer RNAs (tRNAs), and 13 proteins (14). This small molecule has specific characteristics, making it a helpful tool in evolutionary studies (15). The mt-DNA is increased in tissue injury, for example, in sepsis (16-22), trauma (23-26), and malignancy (27-36), indicating severe cellular stress that results in severe cellular death as an essential factor in the release of mtDNA (37). Cell-free circulating mitochondrial DNA (Cfc-mtDNA) has previously been used as a predictive factor for situations associated with the dysfunction of mitochondria, such as cardiac arrest survival (38), dengue severity (39), cancer progression (40, 41), and diabetes mellitus (42). Mutations and replication errors may defect mtDNA. Sufficient mtDNA damage can result in cellular and mitochondrial dysfunction, leading to accelerated aging or impaired health (43).
In this article, we present the association between AF and mtDNA levels and answer whether using mtDNA as a predictive factor for AF is possible.
2. Shared Molecular Basis of Atrial Fibrillation and Mitochondria
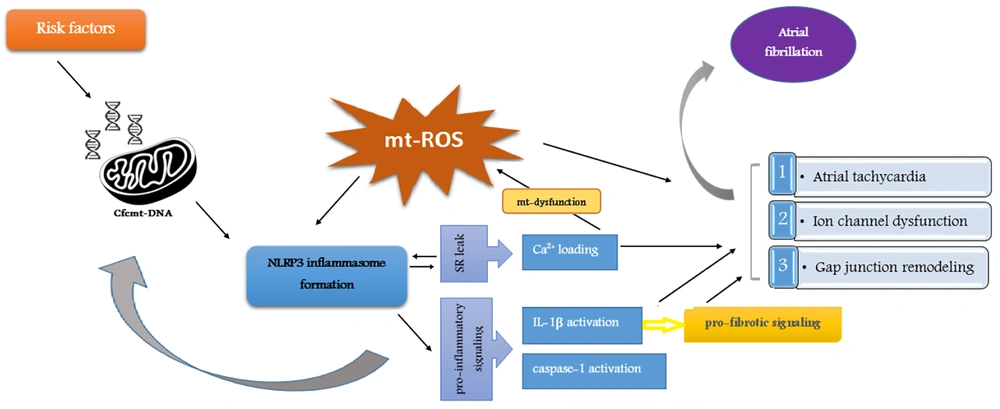
A mitochondrial molecular pathway, including Cfc-mtDNA release, has a significant role in AF pathogenesis (Figure 1). As a damage-associated molecular pattern (DAMP), Cfc-mtDNA is released into the extracellular space during significant cell stress and injury. This phenomenon acts as a signal for other organelles (44). Studies have shown that certain AF risk factors, such as diabetes mellitus and high blood pressure, can lead to mitochondrial dysfunction and a rise in DAMPs (45-50). NLRP3 inflammasome, an innate immune system receptor, regulates the inflammatory response by activating IL-1β. Recent studies have considered NLRP3 inflammasome a potential therapeutic target in inflammatory diseases (51-54). The released Cfc-mtDNA acts as an inflammasome activator (55, 56). On the other hand, the activity of caspase-1 and the inflammatory cytokine IL-1β under the influence of inflammasome cause changes in the mitochondrial membrane and accelerate the release of mtDNA (57, 58). In addition, IL-1β evokes alterations, such as fibrosis, apoptosis, and hypertrophy of cardiac fibroblasts, which are significantly involved in the pathogenesis of AF (59, 60).
Molecular pathways through mitochondria are involved in atrial fibrillation pathogenesis. The formation of NLRP3 inflammasome through the release of Cfc-mtDNA leads to a cascade of events, including Ca2+ loading, IL-1β activation, and caspase-1 activation, which are involved in oxidative stress as an important aspect of the molecular pathogenesis of AF.
Oxidative stress is one of the important aspects of the molecular pathogenesis of AF (61) and is also caused by exposure to AF risk factors and mitochondrial dysfunction (62-64). The mt-ROS activates the NLRP3 inflammasome and Cfc-mtDNA (65, 66). Oxidative stress and mitochondrial dysfunction are enhanced by Ca2+ overload-induced atrial tachycardia. Therefore, Ca2+ overload caused by sarcoplasmic reticulum leak, which is itself promoted by the inflammatory process and NLRP3 inflammasome signaling, accelerates NLRP3 inflammasome formation, disrupts mitochondrial function, and causes more mt-ROS production (61-64, 67-71). In other words, Ca2+ overload provides a platform for initiating and maintaining AF (72).
Taking these together, due to the association of its release with the risk factors of AF and its pathogenesis through mitochondrial signaling pathways, Cfc-mtDNA could be proposed as a promising non-invasive biomarker and a potential therapeutic target for AF. Research has been performed in the field so far, which will be discussed in the following sections.
3. Cfc-mtDNA for Predicting AF, Particularly Paroxysmal AF
The copy number of mtDNA in the peripheral blood was found to be associated with the oxidative stress status in patients. Oxidative stress is also a potential factor contributing to AF pathogenesis (73). Considering this and that a critical modulator of AF in the clinical and experimental setting is mitochondrial dysfunction, scientists came up with the idea that mtDNA may be a potential biomarker predicting AF (74).
In this regard, Zhang et al. measured the mtDNA copy number in the peripheral blood of 485 patients with normal sinus rhythm undergoing cardiac surgery. After surgery, postoperative AF (PoAF) was seen in 101 of 485 patients. The mtDNA copy number in the peripheral blood of these cases was significantly higher than the patients with normal sinus rhythm (36.43 in PoAF patients vs. 16.63 in normal sinus rhythm patients, P < 0.001). They also clarified that mtDNA copy number could predict PoAF acceptable specificity (80.2%) and sensitivity (70.3%) (73).
In another study, Wiersma et al. investigated the association between mtDNA levels in the serum of patients with AF recurrence and AF stage. They evaluated two mtDNA genes, namely ND1 and COX3, in the peripheral blood of 84 control patients, 100 paroxysmal AF (PAF), 59 patients undergoing cardiac surgery not having AF history, 20 long-standing persistent AF (LS-PeAF), and 116 persistent AF (PeAF) patients who underwent either AF treatment (pulmonary vein isolation or electrical cardioversion) or cardiac surgery. According to this study, Cfc -mtDNA levels increased significantly in PAF patients who underwent AF treatment. This means that Cfc-mtDNA levels significantly increased in the early stages of AF (PAF) but decreased in the end-stage AF (LS-PeAF). This study also revealed that Cfc-mtDNA levels in the serum are associated with AF recurrence after all types of treatments, especially in the PVI subgroup. Briefly, this study proved the potential of Cfc-mtDNA levels as an interesting biomarker for the staging of AF, early identification of PAF patients, and identifying patients at high risk of AF recurrence after PVI treatment (75).
In concordance with the above-mentioned studies, Sandler et al. investigated mtDNA at three different time points, including preoperatively, after cardiopulmonary bypass (CPB) within 90 minutes of decannulation, and finally postoperatively on the first and second day after CPB in 16 patients who enrolled prospectively. They found that mtDNA in the blood samples of patients significantly rose after CPB (six-fold elevations after CPB, P = 0.008, and five-fold elevations during 2 days post-surgery, P = 0.02). The PoAF patients showed a rise in the mtDNA levels post-CPB compared to patients without POAF. Moreover, postoperatively, POAF patients showed at least a two-fold elevation of mt-DNA, whereas this increase happened in less than half of patients not having PoAF (P = 0.037). Their results exhibited that the initial tissue damage and following inflammation caused by cardiac surgery played an important role in the development of PoAF, confirming that at the molecular level, there can be a potential mechanistic relation between mtDNA and the development of POAF (76).
Cell-free DNA (CfDNA) has been recently known as a member of DAMPs and has been suggested to have a role in inflammatory disorders. As it has been proven that some inflammatory pathways can lead to AF, Yamazoe et al. (77) aimed to investigate the level of cfDNA as a potential cause of systemic inflammation accompanied by AF. Consequently, they obtained the peripheral blood of 104 non-AF controls and 26 PAF patients and extracted their cfDNA. They noticed that the Cfc-mtDNA copy number is associated with PAF more strongly than nuclear cf-DNA. They also clarified that the unmethylated CpG islands in mt-cfDNA are necessary for promoting a pro-inflammatory response in which IL-1B, IL-6, and toll-like receptor (TLR) 9 play a role. Thoroughly, Cfc-mtDNA activates macrophages and induces pro-inflammatory cytokines via TLR9. Previous studies have reported the association between mt-cfDNA levels and inflammatory cytokines (78-80). They finally concluded that Cfc-mtDNA may play an essential role in such an inflammatory response in this disease. Yamazoe et al. also compared the ability of total cf-DNA, nuclear cfDNA, and Cfc-mtDNA in AF prediction. Their study revealed that Cfc-mtDNA is the most potential biomarker for predicting PAF incidence (77).
Another study investigating the association between mt-DNA levels and AF incidence was conducted by Soltész et al. They assessed mt-DNA levels in the cell-free plasma, peripheral whole blood, and exosome samples. They took peripheral blood from 60 patients with AF and 72 controls. Next, they isolated DNA from plasma and blood, and exosomes were also isolated from the plasma, which was free from cells. Finally, exosome-encapsulated DNA, exoDNA, was extracted, and quantitative RT-PCR was performed. In contrast to the aforementioned studies, they reported no significant difference in mt-DNA levels between cell-free plasma, peripheral blood, and exosomes (81).
4. Cfc-mtDNA and Persistent AF
In a study by Yamazoe et al., 28 PerAF patients were compared with an age-matched control group and a young healthy control group. The Cfc-mtDNA levels in PerAF patients were higher than in both control groups. They also compared n-cfDNA between these groups and observed no significant difference between PerAF patients and the age-matched control group (77). In contrast, Wiersma et al. reported that Cfc-mtDNA levels in the PerAF and long-standing PerAF patients decreased compared to the control group (75). Further studies are necessary to clarify the association between Cfc-mtDNA levels and PerAF.
5. Gender-Related Differences in Cfc-mtDNA
It is commonly known that some gender differences exist in AF (82). Wiersma et al. compared ND1 and COX3 levels between the groups to investigate differences in Cfc-mtDNA levels between female and male patients. They reported no differences in Cfc-mtDNA levels between females and males (75). In line with this study, another research by Soltész et al. found no significant difference in the copy number of mtDNA between genders (81). However, Wiersma et al. found that Cfc-mtDNA levels were significantly elevated in male PAF patients compared to a male control group. In contrast, such differences were not seen in the female PAF patients compared to female controls. These results showed that Cfc-mtDNA levels were associated, especially with male patients with AF (75).
6. Age-Related Differences in Cfc-mtDNA
The Cfc-mtDNA levels are related to the inflammatory status, and there may be a difference between different ages. Therefore, it is necessary to investigate whether age independently affects the Cfc-mtDNA levels. Some studies, such as the one conducted by Soltész et al., suggested no significant difference in the copy number of mt-DNA between different age groups, which agreed with a previous study by Feng et al. (81, 83). In addition, related to the potential role of mtDNA levels in predicting AF, Zhang et al. declared that this novel biomarker is a preoperative potential risk factor for developing AF independent of the patient’s age (73).
7. Conclusions
Our review suggests an association between the serum level of Cfc-mtDNA and AF. Most studies revealed that Cfc-mtDNA serum levels can be used in predicting PoAF and detecting early PAF patients. These studies indicate that age and gender do not affect the results. In addition, studies on Cfc-mtDNA and per-AF have yet to reach similar results, and further research is needed. However, evidence is limited, and future research is necessary to assess the applicability of Cfc-mtDNA as a biomarker for atrial fibrillation.