1. Background
The rate at which heart rate returns to pre-exercise levels is called heart rate recovery (HRR), and a very rapid HHR immediately after exercise is associated with a lower risk of cardiovascular diseases independent of other parameters (1). The change in heart rate during exercise is mainly a function of vagal reactivation and due to the stimulation of the sympathetic autonomic nerves (SANS) and inhibition of the parasympathetic autonomic nerves (PANS). Heart rate after exercise is a specific measure of the functioning of the autonomic nervous system. It is used as a predictive and assessment tool to predict cardiovascular complications and mortality (2). A delay in HRR indicates a decrease in vagal tone or excessive sympathetic activity (3).
Exercise testing is one of the most common and non-invasive tests in the field of cardiology. In most centers, test interpretation is primarily based on ST-segment changes, exercise-induced angina, and exercise capacity. Recently, heart rate changes during and after exercise have emerged as powerful measures of risk. Studies have generally focused on the prognostic implications of increased heart rate during exercise, known as the chronotropic response, and decreased heart rate immediately after exercise or heart rate recovery (4). The decrease in heart rate immediately after exercise is thought to be due to rapid reactivation of the central vagus. Individuals with high levels of parasympathetic activity at rest have a significant reduction in heart rate after exercise, while patients with heart failure show a significant reduction in HHR (5). There is a strong correlation between HHR and the functional capacity of the heart, and as the functional capacity decreases, the possibility of abnormal HHR increases (6).
Since cardiovascular diseases (CVD) are the main complications of delayed HRR and obesity, measurement of obesity indices may be useful to predict changes in HRR and serve as an appropriate screening for high-risk individuals. Weight loss and increased fitness are associated with greater improvements in HRR (7). Several studies have investigated the correlation of post-exercise HRR with some associated factors such as obesity, socioeconomic status, depression, hypertension, diabetes, and metabolic syndrome (8). Also, there is considerable evidence that obesity increases the risk of CVD through multiple mechanisms, including dyslipidemia, hypertension, diabetes, and atherosclerosis (9, 10). Obesity may also cause changes in the balance between sympathetic and parasympathetic autonomic nervous system (ANS) activity (11). Although BMI is traditionally used as a screening tool for obesity, it is better to use tools such as the body fat ratio, which has a better predictive value and predicts the degree of obesity. Heart rate recovery after one minute of exercise is inversely correlated to cardiovascular events and BMI (12, 13).
Although current evidence supports the use of chronotropic failure and HHR for risk stratification, it is currently unclear whether these findings are modifiable risk factors. Therefore, it seems reasonable to examine potential risk factors such as obesity indices in patients and manage such modifiable risk factors in patients with higher risks (5).
2. Objectives
This study aimed to investigate the relationship between BMI and other obesity indices in people referred for exercise testing and HRRT to investigate their autonomic system functions and the risk of cardiovascular diseases.
3. Methods
In this descriptive cross-sectional study, 108 participants were non-randomly and consecutively selected from patients over 18 years of age. They all had an indication for exercise testing and were referred to the exercise testing department of Modarres Hospital, Tehran, Iran, from 2021 to 2022. They did not have CAD or any history of receiving beta-blockers. Besides, patients with valvular heart disease, cardiomyopathy, myocarditis, vasculitis, arrhythmia, and all factors causing termination of exercise testing, such as chest pain and blood pressure drop, were excluded from the study. All participants signed informed consent, and the study protocol was approved by the Shahid Beheshti Medical University Ethics Committee (IR.SBMU.MSP.REC.1400.213).
The patients included in the study were subjected to an exercise test and monitored, and the decrease in heart rate in the first two minutes was recorded. In addition, all demographic and clinical information of the patient, including age, sex, height, weight, waist and hip circumference, and information related to cardiovascular risk factors such as smoking and exercise, history of high blood pressure, high fat, diabetes, and family history of heart disease were recorded based on the questionnaire.
3.1. Statistical Analysis
Data were analyzed using SPSS version 22 software. In the descriptive part, quantitative variables were reported with mean and standard deviation and qualitative variables were reported with number and percentage. For data analysis, Pearson’s correlation test was used for quantitative data and Spearman’s for rank data. In order to compare the groups based on quantitative variables, we first checked the normality of the data distribution using the Shapiro-Wilk test. Then, we used either the Mann-Whitney or t-test to compare the 2 groups. Additionally, we created study charts using PRISM version 8 software. A significance level of P>0.05 was considered for all tests.
4. Results
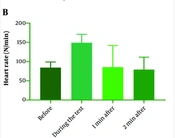
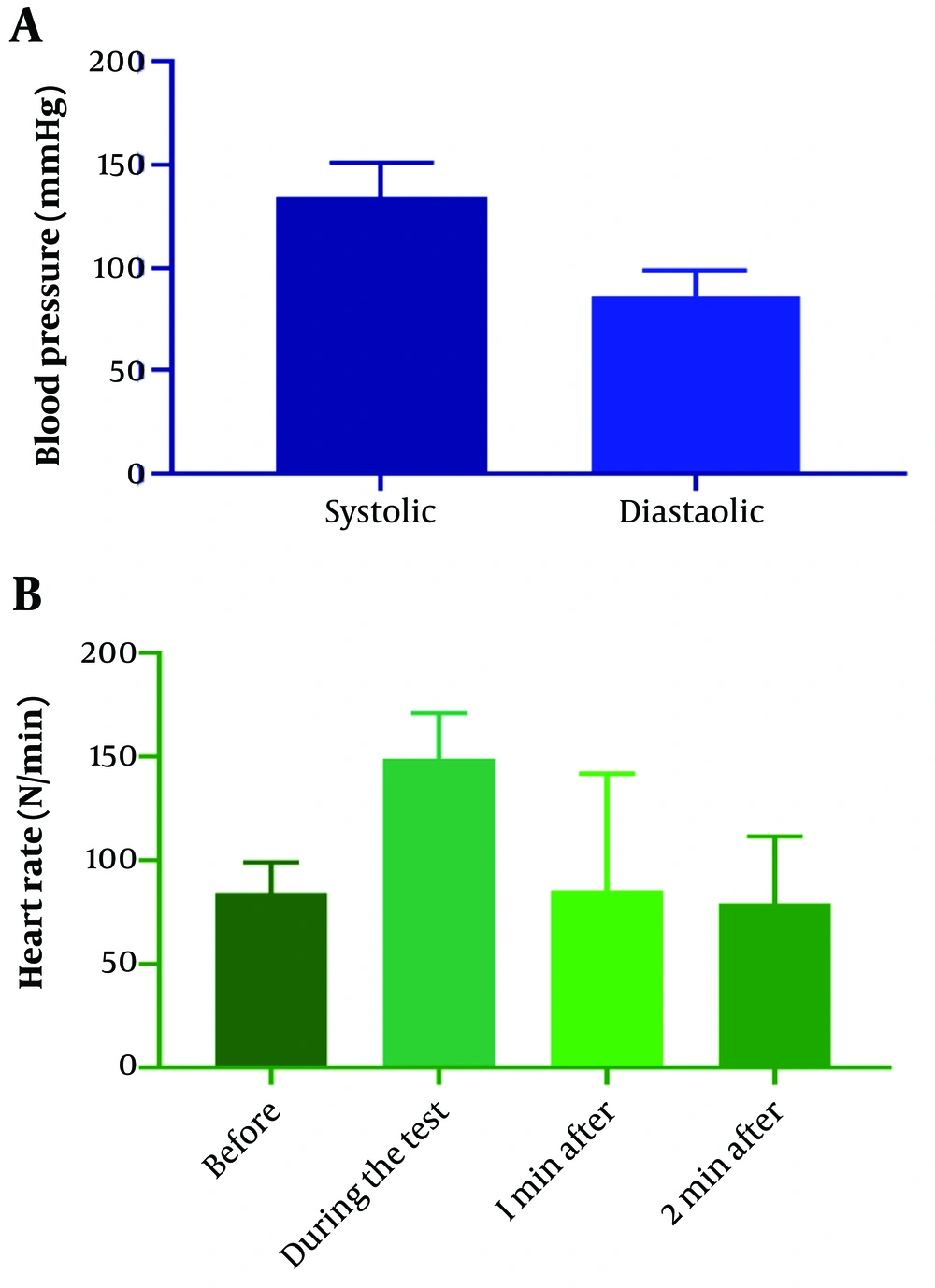
The participants had a mean age of 48.64 ± 12.23 years (ranging from 18 to 80 years), and 72 % were male. Diabetes, hypertension, and hyperlipidemia were diagnosed in 7.5%, 32.7%, and 24.3% of patients, respectively. Approximately 29% of patients had a family history of cardiovascular disease. Other patient characteristics are demonstrated in Table 1. Figure 1 shows the distribution of patients regarding heart rate before, during, one minute, and two minutes after exercise testing.
| Parameter | Min | Max | Mean ± SD |
|---|---|---|---|
| Age (y) | 18 | 80 | 48.64 ± 12.23 |
| Weight (Kg) | 54 | 120 | 80.32 ± 12.47 |
| Height (Cm) | 152 | 187 | 172 ± 8.98 |
| BMI (kg/m2) | 18.04 | 4.04 | 27.12 ± 3.54 |
| Neck circumference (cm) | 31 | 108 | 39.56 ± 7.82 |
| Waist circumference (cm) | 54 | 118 | 95.84 ± 10.01 |
| Hip circumference (cm) | 55 | 126 | 104.29 ± 10.58 |
| WHR | 0.69 | 1.54 | 0.92 ± 0.09 |
| ABSI | 0.04 | 0.09 | 0.08 ± 0.006 |
| ABI | 6.11 | 27.86 | 18.66 ± 3.6 |
| BAI (%) | - 17.62 | - 17.09 | -17.27 ± 0.07 |
| Systolic pressure before the test (mm Hg) | 90 | 180 | 134.4 ± 16.87 |
| Diastolic pressure before the test (mm Hg) | 60 | 156 | 86.38 ± 12.87 |
| Heart rate before the test unit (beats per minute) | 47 | 136 | 84.2 ± 15.33 |
| Heart rate during the test (beats per minute) | 90 | 238 | 149.03 ± 22.1 |
| Heart rate in one minute after the test (beats per minute) | 72 | 190 | 126.22 ± 21.48 |
| Heart rate two minutes after the test (beats per minute) | 53 | 166 | 100.42 ± 17.11 |
| Decreased heart rate in the first minute | 0 | 58 | 22.8 ± 11.71 |
| Decreased heart rate in the second minute | 10 | 123 | 48.61 ± 18.11 |
Demographic Characteristics and Clinical Findings of the Patients
In this study, the correlation of obesity indices such as weight, height, BMI, neck circumference, waist circumference, hip circumference, WHR, ABSI, ABI, and BAI with the reduction of heart rate in the first minute (heart rate during the test - heart rate in the first minute after exercise testing) and the second (heart rate during the test - heart rate in the second minute after the test) was examined after the exercise test. None of the indexes showed a significant correlation with the decrease in heart rate after the exercise test. The results are tabulated in Table 2.
| Parameter | HHR 1 Min After Exercise Test | HHR 2 Min After Exercise Test |
|---|---|---|
| Weight (Kg) | ||
| r coefficient | - 0.07 | 0.05 |
| P-value | 0.45 | 0.59 |
| Height (Cm) | ||
| r coefficient | 0.08 | 0.11 |
| P-value | 0.4 | 0.22 |
| BMI (kg/m2) | ||
| r coefficient | - 0.16 | - 0.04 |
| P-value | 0.08 | 0.64 |
| Neck circumference (cm) | ||
| r coefficient | 0.11 | 0.12 |
| P-value | 0.22 | 0.18 |
| Waist circumference (cm) | ||
| r coefficient | - 0.01 | - 0.1 |
| P-value | 0.85 | 0.28 |
| Hip circumference (cm) | ||
| r coefficient | 0.08 | 0.04 |
| P-value | 0.4 | 0.64 |
| WHR | ||
| r coefficient | - 0.12 | - 0.17 |
| P-value | 0.2 | 0.06 |
| ABSI | ||
| r coefficient | 0.1 | - 0.13 |
| P-value | 0.27 | 0.15 |
| ABI | ||
| r coefficient | - 0.03 | - 0.1 |
| P-value | 0.74 | 0.3 |
| BAI (%) | ||
| r coefficient | 0.04 | 0.001 |
| P-value | 0.62 | 0.98 |
Correlation Assessment Between Obesity Indices and Heart Rate Reduction
Subsequently, the decrease in the heart rate of the patients was investigated in relation to the other factors, including diabetes, high blood pressure, hyperlipidemia, smoking, family history of heart disease, regular exercise, and enough hours of sleep. According to the results, the reduction in heart rate one minute after the exercise test was significantly lower in patients with a family history of heart disease (P-value = 0.01), but it was not associated with insufficient sleep (P-value = 0.06) and exercise (P-value = 0.95). On the other hand, the rate of heart rate reduction two minutes after exercise testing was significantly lower in patients with diabetes, hyperlipidemia, and insufficient sleep (P-value = 0.01, 0.04, and 0.04, respectively). However, there was no significant change regarding hypertension (P-value = 0.24), smoking (P-value = 0.12), exercise (P-value = 0.1), and family history (P-value = 0.6).
5. Discussion
The prognostic significance of HRR after exercise testing as a risk factor for cardiac morbidity and mortality has been shown in different groups of patients and healthy individuals (14-17). Similarly, obesity is a risk factor for cardiovascular disease (18, 19). As shown, weight loss improved HRR in overweight and obese individuals. Therefore, HRR may be a modifiable risk factor through changes in body weight (20). An HRR of 12 beats/min or less is considered the cutoff point for increased CVD risk and mortality (21). Therefore, considering the importance of heart rate reduction after exercise testing, this study was designed to investigate the correlation between obesity indices and heart rate reduction after the exercise test.
Based on the results, none of the obesity indices such as weight, height, BMI, neck circumference, waist circumference, hip circumference, WHR, ABSI, ABI, or BAI had a significant correlation with the reduction of the heart rate first and second minutes after the exercise testing. However, the heart rate reduction one minute after the exercise test was significantly lower in patients with a family history of heart disease. Also, the heart rate reduction two minutes after the exercise test was significantly lower in patients with diabetes, hyperlipidemia, and insufficient sleep.
Contrary to our results, various studies have shown the correlation between obesity indices and heart rate during the exercise test and its reduction after the test. Previous studies have shown the relationship between HRR, BMI, and hip circumference (20, 22). Also, it has been demonstrated that HRR is negatively related to changes in body weight, and this confirms the application of BMI, WC, and WHR in predicting cardiovascular risk (23, 24). In a study of Malaysian male and female adolescents, HRR was inversely correlated with body composition parameters such as BMI, WHR, and body fat (25).
Several studies have demonstrated that obesity alters HR patterns during exercise, and the altered HR response has a marked effect on an individual’s exercise capacity (26). The results of one study demonstrated that BMI was related to HR during the exercise stress test, but resting HR had no significant effect (27). The mechanism behind this inverse relationship between HRR parameters and body fat can be explained by obesity-induced changes in ANS function, which include increased renin-angiotensin complex activity in obese subjects. It has been suggested that obesity may have a negative effect on baroreflex sensitivity (which reduces responsiveness to the shift from sympathetic to parasympathetic) via insulin pathways and disruption of visceral fat production. These mechanisms may also explain why obese subjects may exhibit a higher baseline heart rate at rest than nonobese individuals (28).
The reason for the lack of a significant correlation in our study may be the small sample size. On the other hand, most studies rely on BMI. BMI is the most commonly used anthropometric measure of obesity and is well known for assessing body fat, but other parameters may better estimate body fat composition (29). In addition, the hearts of obese individuals may not respond adequately to autonomic stimuli even in the absence of clinical conditions associated with known morphological and functional changes (30). Therefore, the different heart rate behavior in obese subjects is conventionally attributed to obesity regardless of the patient’s fitness level. The effects of age on HR depend on the balance between vagal and sympathetic, and both decrease with age, and the net effect is variable and generally moderate (31).
Lauer have demonstrated that aging is associated with a reduced number of patients reaching age-predicted target HR during exercise. The vagal response also decreases with age, explaining this decrease in HR recovery. The reduction of exercise tolerance in obese individuals is secondary to increasing age, BMI, sex, and diabetes, but the most important factors that affect exercise performance are chronotropic variables (5). In our study, subjects who showed lower HRR were generally older, but age was not a predictive factor. From age 60 onwards, there is a more rapid decline in parasympathetic modulation, which may be explained by subclinical sinus dysfunction due to changes in calcium channels (32). In our study, a large age range of people was investigated, and a high percentage of patients had a BMI in the normal range, which increases the possibility of scattered findings and reduced correlation.
In line with our study, some studies have not observed a significant correlation between obesity indices and heart rate reduction after the exercise test. However, they had several differences compared to our study. A study that examined the effect of several obesity parameters on cardiac parasympathetic reactivation between obese and normal-fat subjects showed no difference in parasympathetic reactivation using HRR assessment. In addition, this study showed no significant association between parameters of excess fat gain, including BMI, WHR, fat percentage, and trunk fat with HRR (28).
Some studies have found evidence regarding the relationship between clinical factors and heart rate reduction after an exercise test. In these studies, smoking was associated with a lower chronotropic index, even if the smokers were slightly younger and thinner than their non-smoker counterparts (33). Also, diabetes can reduce the chronotropic index, probably because diabetic patients have less norepinephrine release during exercise (34).
Diabetes was associated with a higher risk of HRR impairment in our study. Brinkwort et al., in a prospective study of overweight and obese men following a weight loss program based on dietary restriction without changes in physical activity, found that the best predictors for improvement of HRR were plasma glucose concentration and high blood pressure (20).
Gondoni et al. showed that the HR behavior of obese subjects with or without regular exercise differed from subjects with normal BMI during an exercise test and concluded that obese subjects had a lower HRR regardless of their fitness level (35), which was in line with our findings.
Given the inconsistent findings mentioned in this regard, it is imperative to conduct additional studies with larger and more homogeneous sample sizes to draw conclusive results. One limitation of this study is that the analysis did not consider medications that could affect HR behavior, and the patient’s normal physical activity level was not determined. It is recommended to have a comprehensive evaluation of patients in a large, multicenter-based group, which is prospectively followed by a focused examination of the consequences of obesity. In addition, despite trying to investigate important demographic aspects concerning patients, some determining factors, such as other drugs taken by the patient, have not been taken into account. Also, the lack of a control group matched in terms of age and gender and comparing two groups is one of the limitations of this study.
5.1. Conclusions
Although our study did not show a significant correlation between obesity indices and heart rate reduction after the exercise test, obesity and increased weight have harmful effects on cardiac function and should be considered risk factors that can be addressed, particularly considering the fact that their rates are increasing. The necessity of dealing with this issue has already been highlighted. Therefore, conducting further studies in this field, particularly clinical trials, can illuminate the effects of obesity indices on cardiac efficiency.