1. Background
ST-elevation myocardial infarction (STEMI), which is a subtype of acute coronary syndrome, is defined as the presence of at least one of myocardial ischemia symptoms along with persistent ST-elevation in at least two adjacent ECG, finally leading to myocardial injury or necrosis (1). Despite the significant contribution of STEMI to global mortality and morbidity, factors such as accurate diagnosis, appropriate reperfusion using primary percutaneous coronary intervention (PPCI), antithrombotic therapy, and secondary cardiovascular disease preventive strategies can improve survival after acute STEMI (2-4).
Nowadays, reperfusion therapy by PPCI is the preferred therapeutic option to shrink the size of myocardial infarct (MI) and preserve the systolic function of the left ventricular (LV) following acute STEMI (5-7).
Resolution of ST-segment (STR) and thrombolysis in myocardial infarction (TIMI) and frame count (TFC) are useful parameters to evaluate the reperfusion status following PPCI in STEMI patients (8). Resolution of ST-segment is an inexpensive and non-invasive indicator used for the clinical evaluation of STEMI patients after PPCI. This parameter is visible on ECG, but it does not directly indicate the blood flow (9). In addition, TFC is used to monitor coronary blood flow and is described as the number of cineframes needed for contrast to reach standardized distal coronary landmarks (10). Studies show that incomplete STR and higher TFC are associated with a poor prognosis, high mortality, and low ejection fraction (EF) (11-13).
2. Objectives
Since EF is one of the important parameters of systolic function, we need to understand if this parameter is associated with STR and TFC. It is also important to investigate if this parameter is always a good reflection of the true status of cardiac perfusion pre- or post-PPCI. Therefore, in the present study, we aimed to assess the correlation of EF with TFC and STR in patients with STEMI undergoing PPCI.
3. Methods
3.1. Patients and Study Design
We conducted this investigation on a total of 250 STEMI patients who were admitted to the Shahid Modarres Hospital in Tehran, Iran, and underwent PPCI from 2019 to 2021. According to guidelines, STEMI was defined as at least 30 min and less than 6 hr of evident myocardial infarction chest pain accompanied by ST-segment elevation in two contiguous leads with the cut‐off points of ≥0.2 mV for men and ≥0.15 mV for women during 12–lead ECG (14). Patients were excluded if they had a pacemaker or if ECG showed left bundle branch blocks. All patients included in the study signed an informed consent form. The protocol of the study was approved by the local ethics committee at the Shahid Beheshti University of Medical Sciences.
3.2. Cardiovascular Evaluations
Ejection fraction was evaluated in transthoracic echocardiography before PPCI. Also, EF, along with STR and TFC, was assessed after PPCI in all patients. The ejection fraction was calculated using Simpson’s biplane method following the recommendations of the European Association of Echocardiography and the American Society of Echocardiography (15). Also, ST-segment elevation was examined with calipers to 0.025 mV and 20 ms after the end of the QRS complex with the TP segment as the reference baseline (16). Based on this, patients were divided into two groups: STR less than 50% and STR more than or equal to 50%. TFC was defined as the number of cine-angiographic frames required to reach standardized distal landmarks in ECG, thus providing a quantitative assessment of the epicardial flow. This parameter was established to enhance the reproducibility of angiographic assessments (17, 18). The association of EF before and after the PPCI with STR and TFC was finally examined.
3.3. Statistical Analysis
Continuous variables were evaluated using the t-test and presented as mean ± standard deviation (SD). Categorical variables were assessed by the chi-square test and presented as frequencies. The correlation between quantitative variables, including EF and TFC, was assessed by Pearson correlation, and the association between EF and STR was evaluated by the ANOVA test. Statistical analyses were performed using Prism software version 8. A p-value of <0.05 was considered statistically significant.
4. Results
4.1. Clinical Characteristics and Laboratory Findings
As shown in Table 1, the clinical and demographic characteristics of the patients were compared between the two groups of STR < 50% and STR ≥50%. Our results showed that there was no significant difference between these groups regarding the evaluated characteristics, including clinical and demographic features. Also, there was no significant difference between the two groups in terms of laboratory findings (Table 2).
| Characteristics | STR ≥50% | STR <50% | P-Value |
|---|---|---|---|
| Age, y | 56.81 ± 12.72 | 58.55 ± 8.83 | 0.49 |
| Weight, kg | 81.13 ± 12.74 | 81.92 ± 10.59 | 0.75 |
| Height, cm | 169.01 ± 20.45 | 171.96 ± 5.59 | 0.45 |
| BMI, kg/m2 | 39.11 ± 174.66 | 27.21 ± 3.19 | 0.72 |
| BSA, m2 | 2.05 ± 1.39 | 1.93 ± 10 | 0.67 |
| Syntax score before PPCI | 14.94 ± 6.08 | 16.85 ± 7.19 | 0.13 |
| Sex (male) | 184 | 20 | 0.29 |
| Diabetes mellitus (yes) | 57 | 13 | 0.20 |
| Hypertension (yes) | 110 | 17 | 0.22 |
| Hyperlipidemia (yes) | 77 | 8 | 0.67 |
| Smoking (yes) | 115 | 13 | 0.83 |
| Familial history (yes) | 51 | 4 | 0.46 |
| CKD (yes) | 8 | 0 | 0.99 |
| Obesity (yes) | 41 | 7 | 0.43 |
Abbreviations: CKD, chronic kidney disease; BMI, body mass index; BSA, body surface area.
a Quantitative parameters were mentioned as average ± standard deviation and qualitative parameters as numbers.
| Characteristics | STR ≥50% | STR <50% | P-Value |
|---|---|---|---|
| Neutrophil, % | 77.71 ± 11.48 | 72.96 ± 17.63 | 0.059 |
| Lymphocyte, % | 15.88 ± 15.57 | 39.08 ± 65.19 | 0.077 |
| WBC, 103/µL | 18.56 ± 95.42 | 13.95 ± 5.87 | 0.802 |
| PLT, 103/µL | 237.62 ± 71.46 | 210.55 ± 91.11 | 0.073 |
| Hb, g/dL | 16.93 ± 19.55 | 18.51 ± 23.16 | 0.698 |
| MPV, fl | 9.77 ± 1.05 | 18.16 ± 28.42 | 0.137 |
| GGT, mg/dL | 37.38 ± 51.20 | 43.00 ± 75.50 | 0.625 |
| AST, mg/dL | 208.23 ± 206.50 | 329.58 ± 1006.26 | 0.121 |
| ALT, mg/dL | 78.99 ± 112.09 | 229.42 ± 640.63 | 0.243 |
| ALP, mg/dL | 177.66 ± 65.95 | 152.98 ± 68.38 | 0.073 |
| Total bilirubin, mg/dL | 2.24 ± 10.03 | 24.79 ± 99.84 | 0.261 |
4.2. Correlation of the Pre- and Post-PPCI EF with TFC
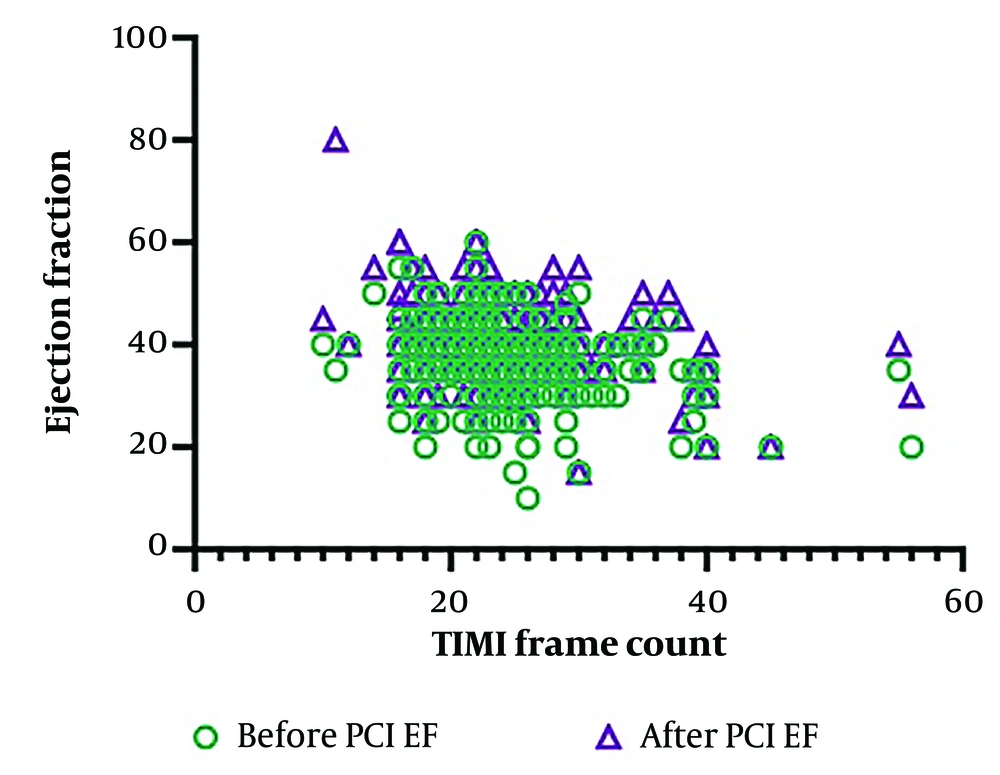
Ejection fraction values before and after PPCI were 36.95 ± 7.77 and 42.10 ± 7.88, respectively. Also, the TFC value after PPCI was 24.27 ± 8.72. Pearson correlation revealed that EF before PPCI showed a weak inverse correlation with TFC (P = 0.0002, r = -0.2336). However, the correlation of EF after PPCI with TFC was stronger (P = 0.0001, r = 0.3137). Figure 1 shows the data distribution regarding the correlation of pre- and post-PPCI EF values with TFC.
4.3. Association of Pre- and Post-PPCI EF with STR
As mentioned above, EF values before and after PPCI were 36.95 ± 7.77 and 42.10 ± 7.88, respectively. Also, in the STR ≥50% group, EF values before and after PPCI were 37.35 ± 7.41 and 33.46 ± 4.87, respectively. On the other hand, in the STR <50% group, EF values before and after PPCI were 42.37 ± 7.21 and 39.8 ± 11.78, respectively. Our results showed that STR (i.e., ≥50% or <50%) was significantly associated with EF before and after PPCI. In this regard, the mean EF in the STR ≥ 50% group was higher compared to the STR <50% group (P-value of 0.0467 and 0.0045 for pre- and post-PPCI EF, respectively).
5. Discussion
It is important to evaluate if EF, as one of the important parameters of systolic cardiac function, is correlated with STR and TFC as parameters representing the actual status of heart perfusion in STEMI patients undergoing PPCI. Therefore, in the present study, we aimed to investigate the correlation between these parameters in patients with STEMI who underwent PPCI. For this purpose, we determined the EF of STEMI patients before and 24 hours after PPCI. We also assessed STR and TFC after PPCI and analyzed their correlation with EF in STEMI patients before and after PPCI.
Our results indicated that EF (either before or after PPCI) was a good reflector of STR value, which represented the incomplete recovery of the microvascular flow to the cardiac tissue after primary PPCI. Also, the correlation of TFC with EF before and after PPCI in STEMI patients was examined. The results showed that there was a weak but significant inverse correlation between these two parameters. However, post-PPCI EF showed a stronger correlation with TFC.
It is notable that STR seems to be a more powerful prognostic factor beyond the recovery of the epicardial blood flow. Therefore, in the early stages of the disease, STR may be a convenient marker to predict the prognosis of AMI patients and must be measured after PPCI (19). In patients with STEMI, microvascular damage due to ischemia can be classified into two types based on its pathophysiology and clinical significance (20). The structural type is due to necrosis, and it is irreversible, while the second type is an obstructive functional abnormality and can be repaired. Early STR could be an important indicator for discriminating between structural and functional microvascular damage (21).
It is difficult to determine the prognosis of microvascular perfusion in low-risk patients with an acceptable EF. Our study showed that EF can be a good indicator of microvascular injury and cardiac perfusion status before and after PPCI. Therefore, both of these parameters can be used as prognostic predictors in STEMI patients.
Park et al. previously reported an association between incomplete STR and reduced EF after PPCI (22). Another study also showed that complete STR in patients with normal EF could indicate a good prognosis in STEMI patients (16, 23). The novelty of our study was in investigating the association of EF and STR before and after PPCI, providing promising insights on low or acceptable EF thresholds.
Research has shown that the relatively lower CTFC of infarcted arteries immediately after PCI can be associated with better improvement in left ventricular function (24-26). Although lower CTFC has been shown to be significantly associated with higher EF, our results did not show a significant correlation between these two parameters, suggesting that the link between these two parameters may be affected by other determinants. On the other hand, this correlation was stronger after PPCI compared to before PPCI, suggesting a decrease in the impact of underlying and temporary variables on these two parameters after PPCI following the improvement of cardiac perfusion status.
5.1. Conclusions
The results of this study showed that EF after PPCI, as an echocardiographic indicator, could provide an overview of the cardiac reperfusion status and microvascular perfusion. We also found that improved cardiac status on ECG could better predict changes in EF.