1. Background
Cardiovascular diseases are among the leading causes of morbidity and mortality in patients with CKD. The majority of patients in stages 3 to 4 of CKD (15 ≤ GFR < 60 mL/min per 1.73 m²) die because of cardiovascular disease rather than progress to end-stage renal disease (ESRD) (1). The exact etiology of cardiovascular disease in CKD patients is unknown. Hypertension (HTN), dyslipidemia, and diabetes, known companions of CKD, are also risk factors for endothelial dysfunction, atherosclerosis, and coronary artery disease (2). Identifying CKD patients at higher risk of adverse cardiovascular events is a crucial step toward lowering the mortality rate. Previous studies have shown that SPECT-MPI is an excellent tool to diagnose or predict adverse cardiovascular events in suspected patients with or without a known history of ischemic heart disease (3, 4). The incremental prognostic value of MPI in CKD patients was reported in some previous studies (5, 6).
2. Objectives
The majority of these studies were small retrospective series. The present prospective study aims to evaluate the predictive value of SPECT-MPI in patients with different stages of CKD.
3. Methods
3.1. Study Population
This single-center prospective study was conducted in the nuclear medicine department of Shahid Labbafinejad Medical Center (Iran) between September 2016 and January 2018. Consecutive CKD patients referred to the department of nuclear medicine for SPECT-MPI due to suspected IHD based on risk factors were included if they were eligible. The inclusion criteria were an established diagnosis of CKD according to the National Kidney Foundation guidelines (7) and age ≥20 years. Patients with a prior history of MI, severe heart disease, or revascularization were excluded. A physician recorded the demographic and clinical characteristics of the patients at the time of the imaging study. The protocol of this study was reviewed and approved by the ethical committee of Shahid Beheshti University of Medical Sciences. All participants signed informed consent.
3.2. SPECT-MPI Protocol
Stress and rest 99mTc-sestamibi SPECT-MPI were performed for all patients according to standard protocols (8). The pharmacological stress test was performed on the first day with Dipyridamole at a dose of 0.14 mg/kg/min over 4 minutes. After 3 minutes, 740 MBq of 99mTc-MIBI was injected intravenously, and 30 minutes later, a heart scan was performed. The rest phase of the imaging was completed on the second day. For this purpose, all patients received 555 MBq of 99mTc-MIBI, and 45 minutes later, imaging was obtained. The images were acquired on a Siemens single-head SPECT camera, and a Butterworth filter (Cutoff 0.3, Order 2.5) was applied for image reconstruction. QGS software calculated SSS, summed rest score (SRS), and summed difference score (SDS). MPI studies were further classified as normal (SSS 0 - 3), mildly abnormal (SSS 4 - 8), moderately abnormal (SSS 9 - 13), and severely abnormal (SSS ≥ 14). Similarly, based on SDS, the ischemic burden was categorized into no ischemia (SDS 0 - 1), mild ischemia (SDS 2 - 4), and moderate to severe ischemia (SDS ≥ 5) (9).
Two independent nuclear medicine specialists interpreted all imaging data.
3.3. Follow-up and Outcomes
All patients were contacted one year after the baseline evaluation. The primary outcome measure was the incidence of cardiac events, defined as cardiac death, MI, heart failure, and any cardiac intervention (CI).
3.4. Statistical Analysis
We used the Statistical Package for the Social Sciences (SPSS) version 25 to perform all statistical analyses in this study. The chi-square test compared categorical variables. An independent samples test was used to evaluate the differences between the study groups regarding the continuous variables. Logistic regression with the stepwise forward method was performed to identify independent predictors of cardiac events. Patients' demographic, clinical, and MPI data were included as covariates. P < 0.05 was considered significant.
4. Results
4.1. Patient Characteristics
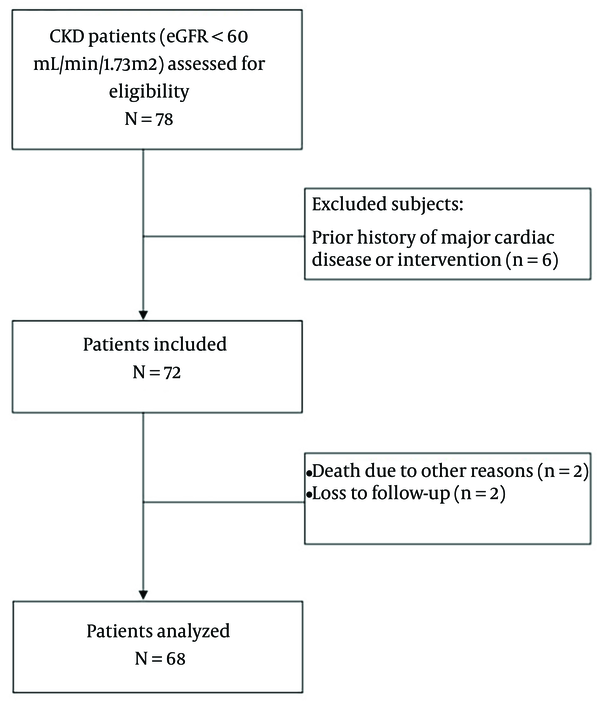
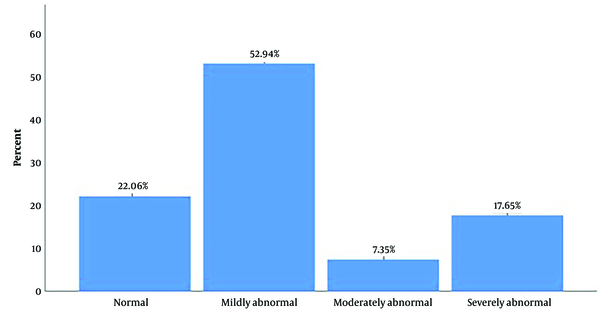
A total of 68 patients were included (Figure 1). The baseline characteristics of the patients are summarized in Table 1. Abnormal MPI was detected in 53 (77.3%) patients. The severity of perfusion defects in patients is demonstrated in Figure 2.
| Characteristics | All Patients | CI-Free Group | CI Group | P-Value |
|---|---|---|---|---|
| 68 | 50 | 18 | ||
| Age | 57.3 ± 11.1 | 56.6 ± 10.2 | 59.4 ± 13.3 | 0.364 |
| Male | 32 (47.1) | 24 (48) | 8 (44.4) | 1.00 |
| Smoking | 14 (20.6) | 12 (24) | 2 (11.1) | 0.323 |
| Hemodialysis | 29 (42.6) | 24 (48) | 5 (27.8) | 0.171 |
| Diabetes mellitus | 36 (52.9) | 22 (44) | 14 (77.8) | 0.026 |
| Hypertension | 37 (54.4) | 33 (66) | 4 (22.2) | 0.002 |
| Dyslipidemia | 18 (26.5) | 17 (34) | 1 (5.6) | 0.027 |
| EF | 53.3 ± 6.8 | 54.9 ± 5.7 | 48.6 ± 7.6 | 0.004 |
| Serum Cr | 4.6 ± 2.7 | 4.6 ± 2.8 | 4.7 ± 2.3 | 0.952 |
| eGFR | 22.4 ± 13.9 | 23.2 ± 14.9 | 19.7 ± 10.2 | 0.367 |
| MPI data | ||||
| Abnormal MPI | 53 (77.9) | 35 (70) | 18 (100) | 0.007 |
| SSS | - | 4.96 ± 1.2 | 11.1 ± 2.2 | < 0.001 |
Baseline Characteristics of the Patients a
4.2. Follow-up Results
Within one year, 20 (29.4%) patients underwent coronary angiography, and cardiac intervention was performed for 18. The baseline clinical characteristics of the CI-free and CI groups are compared in Table 1. The two groups were comparable regarding age, gender, smoking rate, hemodialysis rate, serum Cr, and eGFR. The frequency of diabetic patients was significantly higher in the CI group. The mean ejection fraction (EF) was statistically lower in these patients. Hypertension and dyslipidemia were more frequent among the CI-free group. All patients in the CI group had abnormal MPI results, and compared to the CI-free group, they had significantly higher SSS.
4.3. Predictors of CI
According to multivariate logistic analysis, SSS was a significant predictor of CI (Wald = 5.99, OR = 12.56, SE = 1.03, P-value = 0.014). None of the other included variables were significant.
5. Discussion
Here we assessed the predictive value of MPI-SPECT for predicting cardiac events in patients with CKD. MPI was abnormal in 77.9% of the patients. During the one year of this study, 26.5% of the patients became candidates for CI due to abnormal coronary angiography. All patients within the CI group had abnormal MPI. The results of this study confirm previous studies that concluded abnormal MPI is associated with poor cardiovascular prognosis in patients with impaired renal function (10). The predictive value of MPI was evaluated in a cohort study with 303 ESRD patients. The authors demonstrated a cumulative risk of abnormal MPI on adverse cardiac events (11). Several previous studies have shown the cardiac diagnostic and prognostic value of SSS in patients with CKD (12, 13). In line with these studies, we demonstrated that patients in the CI group had significantly higher SSS. SSS was the only significant predictor of CI. A single increase in SSS was associated with a 12.56 times greater risk of CI. Likewise, in another study, higher SSS was observed among patients who developed cardiac death within one year of the study (14). In a study by Nakajima et al., similar to our survey, factors like LVEF and eGFR were investigated and found to be significantly lower in the group that experienced a cardiovascular event, such as sudden death, cardiac death, or death due to heart failure, compared to the group that did not experience a cardiovascular event. Additionally, parameters like SRS and SSS were significantly higher in the cardiovascular event group, but SDS was not (15).
Nakamura et al. have shown that the incidence of cardiac events was 2.6 times more common among CKD patients with SSS > 8. They also reported a 9% higher risk of cardiac events following an increase in SSS (16). In a study by Kasama et al., SSS was shown to be a significant predictor of cardiac death in 299 patients with different stages of CKD 14. Bhatti et al. have shown that the annualized rate of cardiac death was several times higher among patients with SSS ≥ 4 and GFR < 60 (17). Here we showed that patients in the CI group had significantly lower EF. Diabetes was also more prevalent among the CI group. Surprisingly, dyslipidemia and HTN were more common in the CI-free group. However, none of these variables were significant predictors of CI in the final model.
In contrast to our findings, GFR, diabetes, HTN, dyslipidemia, and EF were reported to have significant predictive value for cardiac events in patients with abnormal MPI, which seems to be due to the larger sample size of these studies compared to ours (12, 17, 18). In a retrospective cohort study in Japan, 529 CKD patients without a diagnosis of coronary artery disease were included. After one year of follow-up, 33 patients died due to cardiovascular events. Hypertension, unlike dyslipidemia, was more common in non-survivors, and similar to our results, SSS was significantly higher in the cardiac events group in univariate and multivariate analysis (19).
Yoda et al. retrospectively reviewed data obtained from 2243 CKD patients who underwent stress MPI (18). Compared to the event-free group, male gender, chest pain, history of prior MI, and diabetes were more common among patients within the cardiac event group. Moreover, SSS was higher, and EF and eGFR were lower in this group. The authors identified diabetes, eGFR, SDS, and SSS as independent predictors of cardiac events in these patients. Kasama et al. found that SDS and eGFR are negatively associated with significant adverse cardiac, cerebrovascular, and renal events. Similar to our results, they did not report any significant predictive value of diabetes, EF, HTN, and dyslipidemia for cardiac events (14).
The majority of previous studies were retrospective, and they evaluated the significance of abnormal MPI in both CKD and non-CKD patients. Thus, the prospective design and focused study population are notable advantages of this study. The small sample size and short follow-up period are major limitations of the present study. We did not detect any cardiac death, MI, or heart failure during the study period.
5.1. Conclusions
Therefore, it was not possible to evaluate the prognostic value of MPI for predicting major cardiac events in our CKD patients. Due to the small sample size, this study's statistical analyses are underpowered. To better understand the prognostic value of MPI in CKD patients, future prospective studies with larger sample sizes and longer follow-up durations are needed.