1. Introduction
Hoarseness is usually caused by unilateral damage to the recurrent laryngeal nerves. The left recurrent laryngeal nerve is longer than the right, making it more vulnerable to damage (1, 2). Ortner’s syndrome, described by Nobert Ortner in 1897 in patients with mitral stenosis and left atrial enlargement, can also result from other causes such as thoracic aortic aneurysm, pulmonary artery enlargement, hypertension, and aberrant subclavian artery (3). Its exact prevalence is unknown but appears to be very rare. It is more prevalent in men and the elderly, though it can also be seen in infants (4-6).
The management of Ortner’s syndrome caused by aortic aneurysm includes four options: Surgery, thoracic endovascular aortic repair (TEVAR), hybrid intervention (surgery + TEVAR), and conservative management. Conservative management has the poorest prognosis, while surgery and TEVAR show the best outcomes for symptom relief in the short-term follow-up (5, 7, 8). Although TEVAR is minimally invasive and has a lower risk of morbidity and mortality compared to open surgery, reducing the patient's length of stay in the hospital and post-procedure recovery time, it does have its own complications. The most significant complications include stroke, spinal cord ischemia, acute renal failure, dissection of the aorta or its branches, aortoenteric or aortobronchial fistula, infections, and mesenteric ischemia. Stroke can occur following TEVAR in up to 7% of cases and may be fatal in 30% of those instances. Brain ischemia complications are often silent and asymptomatic but can increase the risk of future cerebrovascular accidents (CVA) (9, 10).
A well-known type of CVA is a posterior territory stroke due to left subclavian artery (LSA) involvement, which is six times more common than anterior territory strokes and has a poorer outcome. LSA is obstructed in about one-third of TEVAR cases, leading to the development of techniques to prevent CVA arising from this cause. One commonly described method is the left carotid to left subclavian artery bypass, which allows blood to pass from the carotid to the subclavian artery and then to the vertebral artery. This approach may prevent posterior circulation ischemia in patients with vertebrobasilar insufficiency but cannot prevent embolic CVA during the procedure, a more prevalent cause.
Left subclavian artery occlusion may occur during the procedure. In this scenario, the interventionist must attempt to recanalize the artery using pharmacologic (thrombolytic) or mechanical methods (such as mechanical embolectomy) and may seek vascular surgery consultation. There is no universally accepted management for LSA occlusion yet. Some experts perform routine LSA revascularization, while others do so only when patients become symptomatic. If the patient has a dominant left vertebral artery, found in 2 - 8% of patients undergoing TEVAR, routine prophylactic LSA revascularization is strongly recommended. Pre-procedural evaluation of the patency of the circle of Willis may also play a role in prophylactic revascularization (11).
2. Case Presentation
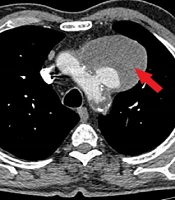
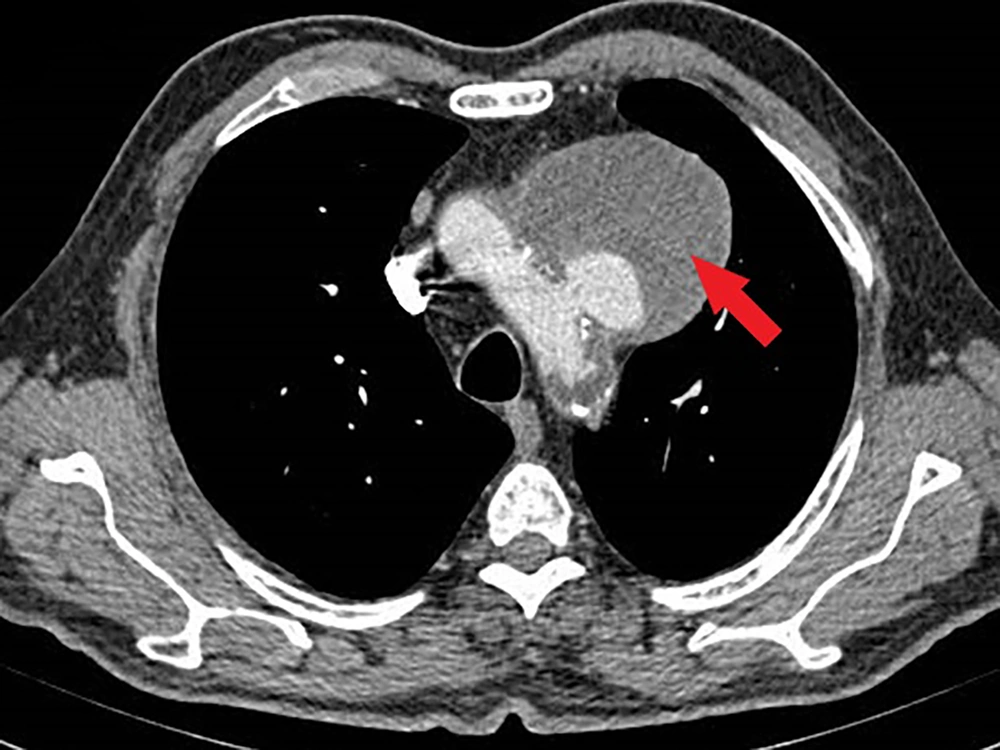
A 62-year-old male smoker with a history of hypertension and dyslipidemia experienced hoarseness for over six months. He was evaluated by otorhinolaryngologists and underwent fiberoptic laryngoscopy, which revealed fixation of the left true vocal cord. Subsequently, a spiral head and neck CT scan and chest X-ray (CXR) were performed. The head and neck CT scan showed no unusual findings, but the CXR revealed a significant mass in the upper mediastinum. A spiral chest CT scan with contrast was conducted for further evaluation, revealing a large aortic arch pseudoaneurysm (neck: 25 mm, open lumen: 23 × 35 mm, total size: 82 × 65 mm) likely compressing the left recurrent laryngeal nerve, causing left true vocal cord palsy and hoarseness. The patient was then referred to our center for symptomatic aortic arch aneurysm treatment.
Upon initial physical examination, the patient had nearly equal blood pressure in both upper extremities (right: 150/90 mmHg, left: 140/95 mmHg). All distal limb pulses were present and symmetric. Heart auscultation revealed no abnormal murmurs. The patient's ECG showed normal sinus rhythm and normal axis but ST-segment depression in V4 - V6, although he reported no chest pain. We reviewed the patient’s chest imaging, which revealed a saccular aneurysm in the aortic arch (Figure 1), with no signs of dissection or rupture. The patient's echocardiography indicated normal left ventricular function (LV ejection fraction = 55 - 60%). Additionally, the patient had mild left ventricular hypertrophy, mild mitral annular calcification, mild mitral and tricuspid regurgitation, grade I diastolic dysfunction, and a mildly dilated ascending aorta (39 mm). The aortic arch aneurysm and its thrombosis were also visible on echocardiography.
Doppler sonography of the carotid arteries showed an uncomplicated plaque on the posterior side of the left carotid bulb without significant stenosis.
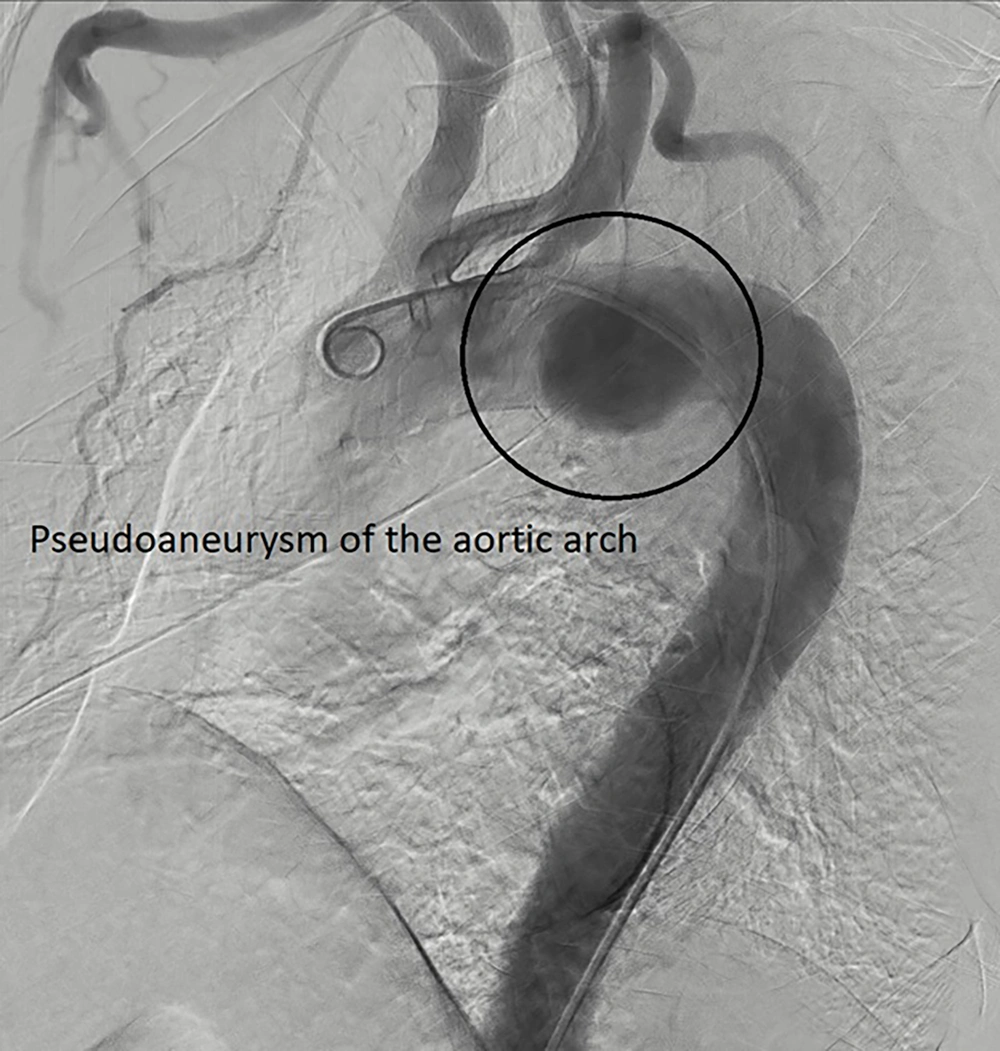
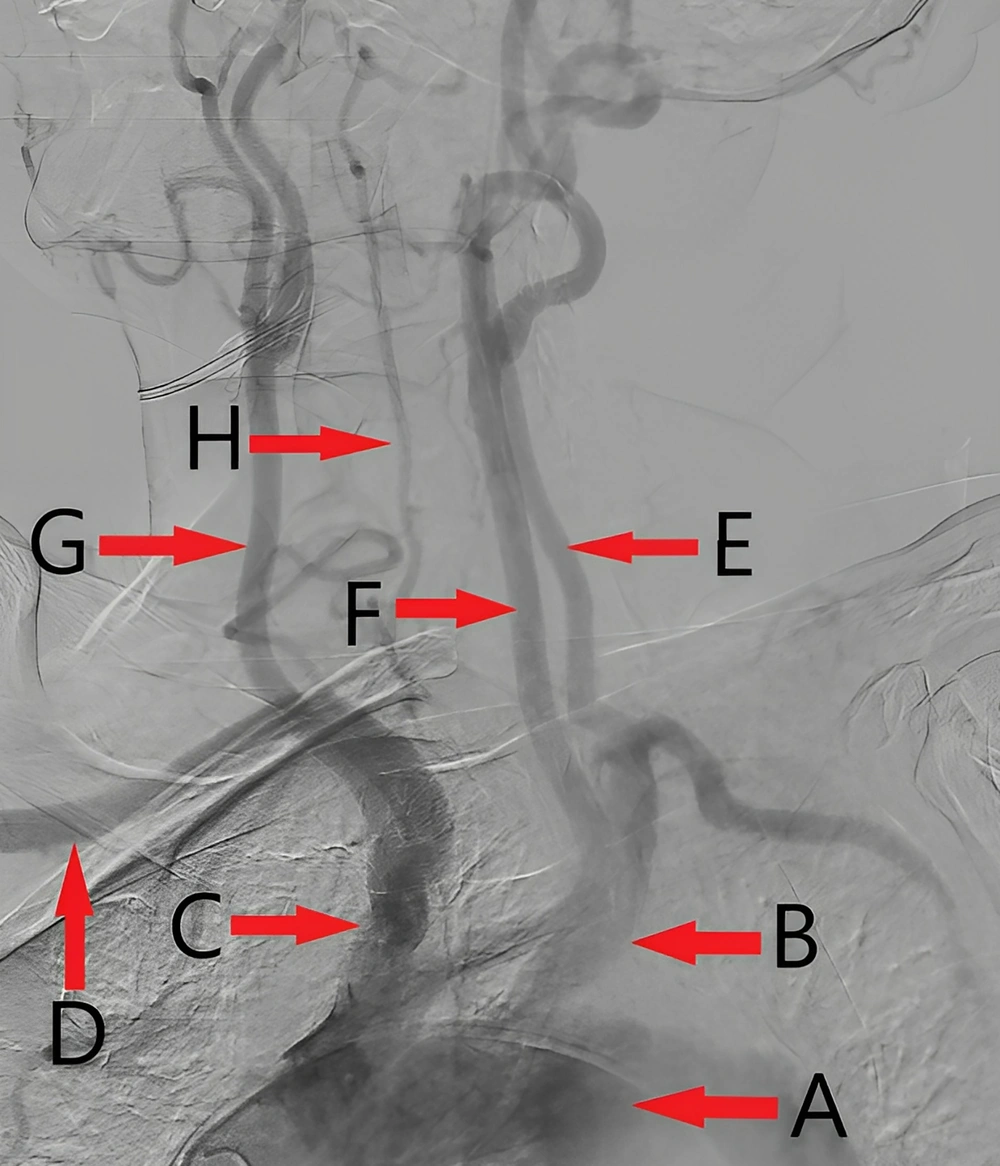
The patient then underwent coronary angiography and aortography, revealing a 2-vessel coronary disease with moderate stenosis in the mid part of the left coronary artery, and a proximal occlusion of the right coronary artery. Due to the patient's asymptomatic condition, a medical follow-up was planned. Aortography confirmed a large pseudoaneurysm in the aortic arch (Figure 2), with patent carotid and iliofemoral arteries, and a mild plaque in the ostium of the left subclavian artery. Notably, the right vertebral artery was diminutive (Figure 3), which is an absolute indication for prophylactic LSA revascularization, as discussed later.
Given these findings, the initially planned TEVAR procedure was canceled, and the patient was scheduled for vascular surgery. However, the patient refused the surgery, and no follow-up data is available.
3. Discussion
The escalating prevalence of cardiovascular disease risk factors such as diabetes mellitus, hyperlipidemia, and smoking contributes to the development of cardiac conditions necessitating medical intervention, with a concomitant need to acknowledge potential complications (12-16). There is no general consensus on managing an asymptomatic compromised artery during TEVAR, but there are some absolute indications for prophylactic LSA revascularization. These include a patent left internal mammary artery (LIMA) supplying the coronary artery (usually the LAD) in post-coronary artery bypass graft (CABG) patients, any discontinuity in vertebrobasilar artery collaterals such as early termination of the left vertebral artery in the posterior inferior cerebellar artery, an absent or diminutive right vertebral artery, a functional arteriovenous shunt in the left arm used for hemodialysis, a history of infrarenal aortic repair with lumbar and middle sacral artery ligation, planned long-segment coverage of the descending thoracic aorta (over 20 cm) that may cover the origin of intercostal arteries, occlusion of the hypogastric artery, and planned future interventions in the distal thoracic aorta for aneurysmal formation (17). Previously, the TEVAR procedure was preferred for high-risk patients with several comorbidities, but with the rapid progression of TEVAR technology, this procedure can now be performed safely even in low-risk patients. The relief of hoarseness after open surgery or the TEVAR procedure is variable; however, patients treated with TEVAR experience faster recovery from hoarseness.
Brain ischemia is a common finding in post-TEVAR procedure patients. A study shows that every patient undergoing the TEVAR procedure experienced brain ischemia, as detected by Transcranial Doppler (TCD) (11). This could be due to embolic events or hypoperfusion of the brain caused by compromised brain-supplying arteries. In recent years, there has been a growing emphasis on neuroprotective strategies during TEVAR to reduce the risk of cerebral ischemia. One such strategy involves using pharmacological agents like antiplatelet drugs and statins to minimize the risk of embolic events. Additionally, intraoperative neuromonitoring techniques, such as transcranial Doppler ultrasound, provide real-time feedback on cerebral blood flow, allowing for the early detection and intervention of potential ischemic events. These approaches, combined with meticulous surgical techniques and careful patient selection, form the cornerstone of a modern, multifaceted strategy for preventing cerebral ischemia during TEVAR.
Another significant advancement is the progress in endovascular devices and techniques. The use of custom-made fenestrated and branched stent grafts enhances the preservation of blood flow to critical arteries, such as the left subclavian artery, without requiring extensive surgical intervention. This approach not only reduces the incidence of perioperative stroke but also improves overall patient outcomes. Additionally, preoperative planning with advanced imaging modalities like 3D CT angiography allows for precise mapping of the aortic arch and its branches, facilitating a more tailored and safer approach for each patient. These innovations highlight the importance of an integrated strategy that combines medical, surgical, and technological advancements to improve the safety and effectiveness of TEVAR procedures (18).
There is no universally accepted management strategy for preventing and treating brain ischemia during TEVAR. Some experts advocate for routine LSA revascularization using a hybrid procedure in all cases. Others recommend routine LSA revascularization in all cases except those with a dominant right vertebral artery or an absent left vertebral artery. Some suggest prophylactic LSA revascularization only for patients with a dominant left vertebral artery, while many prefer revascularization only for those who become symptomatic during the procedure. There are controversial results regarding the impact of prophylactic LSA bypass in preventing CVA, and there is a lack of evidence supporting its superiority.
Given these uncertainties, some simple measures can help prevent brain ischemia before and during the TEVAR procedure. Firstly, the medical team should carefully evaluate the arteries supplying the brain and assess the need for prophylactic LSA revascularization. The operator should be particularly cautious with patients who have a shaggy or calcified aorta or who might have an ulcerative plaque in the aortic wall. Minimizing catheter movement to only necessary maneuvers and being gentle can help prevent plaque embolization (18).