1. Background
Coronavirus disease 2019 (COVID-19), which emerged in December 2019 in Wuhan, China, is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This pandemic spread worldwide and resulted in nearly 4.3 million deaths. Based on investigations in China, patients with previous cardiovascular disorders have a higher rate of mortality and morbidity from COVID-19 (1, 2). Symptoms include fever, shortness of breath, and cough, with less common presentations such as anosmia, sore throat, anorexia, dysgeusia, nausea, myalgias, malaise, and diarrhea (3). It has been observed that cardiovascular conditions such as clot formation, cardiogenic shock, heart failure, and acute coronary syndrome (ACS) may occur with higher incidence after COVID-19 infection (4). Thrombus formation due to cardiovascular conditions in such patients can be visualized easily on two-dimensional echocardiography (ECG) (5).
Although acute respiratory failure is indicated as the leading cause of death in COVID-19 patients, cardiovascular conditions including arrhythmia, myocardial injury (6), ACS, and thromboembolism may also contribute to mortality through the development of ischemic or non-ischemic cardiac injury. Thromboembolic events have been repeatedly described as a problem in patients with COVID-19 infection. The underlying mechanism for thrombus formation in these patients is proposed to be the activation of endothelial cells by viral particles. Incidence rates of clot formation are reported to be approximately 2.6% in hospitalized non-critically ill patients, while in critically ill patients, the incidence is much higher at 35.3% (7). Throughout the COVID-19 pandemic, the occurrence of left ventricular thrombus (LVT) among patients has been reported to be increased.
Myocardial injuries, especially thrombotic events, occurred in a large portion of hospitalized COVID-19 patients (8, 9), which were associated with a higher risk of morbidity and mortality in these patients. There are several theories regarding the mechanisms of thrombus development, one of the most common problems of COVID-19 infection (10). Several factors contribute to a higher incidence of thrombotic events in COVID-19 patients, including viral-stimulated inflammatory activity, COVID-19-induced hypoxia, COVID-19-induced myocarditis, viral-stimulated production of neutrophil extracellular traps, antiphospholipid antibody syndrome, post-COVID-19 increased incidence of Takotsubo cardiomyopathy, prolonged hospitalization of patients, and COVID-19 associated HIT (11-17).
In our previous investigation, routine cardiac examinations of patients presented with COVID-19 revealed left ventricular thrombi (LVTs) in individuals with normal myocardial motion on ECG and normal coronary results on noninvasive cardiovascular diagnostic examinations. Such thrombi were commonly asymptomatic or may cause heart failure (18).
2. Objectives
The present investigation was designed to investigate the risk factors and outcomes of COVID-19 patients who had presented with an LVT.
3. Methods
This observational investigation was conducted at Taleghani Hospital (Tehran, Iran) from March 2020 to September 2020. Data of COVID-19 patients, including demographic information, presenting symptoms, risk factors, home medications, chest radiography evidence, laboratory test results, echocardiographic findings, medications received, and outcomes after one-month follow-up, were collected from electronic records. We included 22 COVID-19 patients aged between 18 and 65 years who were admitted to the hospital for COVID-19 infection. Diagnosis of SARS-CoV-2 infection in patients was confirmed by real-time polymerase chain reaction (real-time PCR) assay performed on specimens collected from nasal or pharyngeal swabs or serologic testing.
Additional inclusion criteria were the absence of cardiac symptoms, no evidence of myocardial injury, normal coronary results confirmed via noninvasive examinations, normal myocardial motion in two-dimensional echocardiography (2D-ECG), presence of a LVT on 2D-ECG, and resolution of the thrombus with anticoagulants during hospitalization (confirmed by follow-up 2D-ECG). Patients who experienced cardiovascular or neurological issues or who died during hospitalization due to the infection were excluded. Additionally, patients with cardiovascular conditions such as valvular, coronary, and congenital heart disorders, a history of cardiac surgeries or interventions, a history of deep vein thrombosis (DVT) or peripheral thromboembolism, and a previous hypercoagulable state were excluded from the study.
The present investigation was conducted in accordance with the principles of the Declaration of Helsinki and adhered to the regulations of the Shahid Beheshti University of Medical Sciences ethical committee (IR.SBMU.RETECH.REC.1401.525).
Quantitative parameters were reported as mean ± standard deviation (SD), while qualitative parameters were expressed as absolute frequency and percentage. All statistical analyses were performed using SPSS software version 24.
4. Results
4.1. Baseline Characteristics
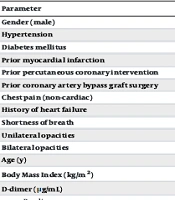
A total of 22 patients were included in the study, and their demographics, clinical, and laboratory characteristics are presented in Table 1. The mean age of the participants was 49 years, ranging between 18 and 65 years. Fourteen (63.5%) patients were male. Upon admission to the hospital, patients did not exhibit any cardiac symptoms. The mean duration of hospitalization for the included patients was 14 days, ranging from 7 to 23 days. Hypertension and diabetes mellitus were prevalent among the patients, with hypertension being the most common comorbidity present in 10 (45.4%) patients, and diabetes mellitus reported in 29% of patients.
Plasma levels of high-sensitivity cardiac troponin T and D-dimer were elevated compared to baseline. High-sensitivity cardiac troponin T was elevated to 9.4 ng/L compared to the mean level at admission of 6.2 ng/L in 22 (33.8%) patients. D-dimer levels were also higher at 3.5 μg/mL compared to baseline levels of 0.9 μg/mL (Table 1).
| Parameter | Values a |
|---|---|
| Gender (male) | 14 (63.5) |
| Hypertension | 10 (45.4) |
| Diabetes mellitus | 6 (29.0) |
| Prior myocardial infarction | 0 (0) |
| Prior percutaneous coronary intervention | 0 (0) |
| Prior coronary artery bypass graft surgery | 0 (0) |
| Chest pain (non-cardiac) | 3 (13.6) |
| History of heart failure | 0 (0.0) |
| Shortness of breath | 12 (54.5) |
| Unilateral opacities | 4 (18.1) |
| Bilateral opacities | 15 (68.1) |
| Age (y) | 49 (18 - 65) |
| Body Mass Index (kg/m2) | 26.5 (24.3 - 31.2) |
| D-dimer (μg/mL) | |
| Baseline | 0.9 (0.3 - 1.3) |
| Peak | 3.5 (0.6 - 0.5) |
| Cardiac troponin I (ng/mL) | |
| Baseline | 0.0 (0.0 - 0.0) |
| Peak | 0.01 (0.0 - 0.02) |
| Cardiac troponin T (ng/mL) | |
| Baseline | 0.0 (0.0 - 0.0) |
| Peak | 0.0 (0.0 - 0.0) |
| High-sensitivity cardiac troponin T (ng/L) | |
| Baseline | 6.2 (3.0 - 9.4) |
| Peak | 9.4 (5.6 - 14.8) |
| CK-MB (ng/mL) | |
| Baseline | 1 (0.7 - 2.2) |
| Peak | 1.1 (0.6 - 2.9) |
a Values are expressed as No. (%) or mean (range).
4.2. Outcomes
Table 2 displays the outcomes observed in patients after one-month follow-up. Of the 22 patients surveyed, 9 (40.8%) were admitted to the ICU, and 4 (18.1%) died during follow-up. Mechanical ventilation was required for 6 (27.2%) patients. Acute respiratory distress syndrome (ARDS) and acute kidney injuries were reported in 6 and 5 patients, respectively. Approximately 27% of patients (6 individuals) experienced shock, and ventricular arrhythmias (VAs) were reported in only one patient.
| Outcome | Values a |
|---|---|
| Death | 4 (18.1) |
| ICU admission | 9 (40.8) |
| Mechanical ventilation | 6 (27.2) |
| Acute respiratory distress syndrome | 6 (27.2) |
| Acute kidney injury | 5 (22.7) |
| Shock | 6 (27.2) |
| Ventricular arrhythmias | 1 (4.5) |
a Values are expressed as No. (%).
5. Discussion
Patients with COVID-19 may be predisposed to arterial and venous thromboembolic disorders due to factors such as hypoxia, diffuse intravascular coagulation, and excessive inflammation (9). While the mechanism of coagulation activation induced by SARS-CoV-2 infection remains unclear, it is proposed to be correlated with an amplified inflammatory response (19). The association of anticoagulant drugs with better outcomes in hospitalized individuals with COVID-19 has been noted (20).
In a meta-analysis, Chi et al. observed that the incidence of DVT in COVID-19 patients was 23.9%, even after receiving anticoagulation (21). Conversely, the occurrence of arterial thrombosis is much lower compared to venous thromboembolism. Only a small number of LVT cases associated with COVID-19 have been identified in the literature, most of which were accompanied by myocardial infarction (MI) (22, 23). Coronavirus disease 2019 can be associated with an excessive inflammatory response leading to impaired coagulation system activation and manifestations of vasculitis in small vessels and severe microvascular thrombosis (24, 25).
Since the prevalence of VAs is higher in cases with complicated hospitalization, these are proposed as a marker for severe systemic disease (26, 27). The overall occurrence of VAs in patients with COVID-19 varies from 0.15% to 8.7%, which is probably due to differences in the definition of VAs and analyzed populations (26, 28). However, the independent association of VAs with the mortality rate in patients with COVID-19 remains unclear. A review by Philip et al. revealed that the overall mortality rate in COVID-19 patients with LVT was 23.1% (29). Another study indicated a mortality rate between 1.1% and 2.0% in COVID-19 patients, and the incidence of venous thromboembolisms (VTEs) in these patients was associated with a higher risk of mortality (30).
In China, after conducting a cohort study on 81 COVID-19 patients, it was reported that the prevalence of VTE in the study population was 25% (31). While a study in Germany reported a cumulative occurrence of 49% for VTE in ICU-admitted patients with COVID-19 (32), another study stated an incidence rate of 34% symptomatic VTE in a cohort with a smaller study population (33). In the present study, we only included patients diagnosed with VTE. Future investigations are needed to evaluate the prevalence of VTE in COVID-19 patients in our population.
One of the other important risk factors for VTE development is prolonged mechanical ventilation (34). A study on VTE in ICU-admitted patients with COVID-19 reported 8 days as the mean length of mechanical ventilation. Mechanical ventilation decreases blood flow to the heart, which may lead to the formation of a DVT by accelerating the stasis of venous blood (35). In our study, mechanical ventilation was performed for approximately 27% of the study population. Such a high prevalence may confirm the role of mechanical ventilation in VTE development.
Acute respiratory distress syndrome is another risk factor for the development of VTE, which may cause a bigger risk of VTE and pulmonary embolism in ICU-admitted COVID-19 patients (36). Indeed, VTE is frequently reported in COVID-19 patients who received medications for ARDS, and it was also associated with a high death rate (37).
Coronavirus disease 2019 patients are reported to have encountered acute kidney injury (AKI) with a prevalence rate of 0.5% to 35%, which is correlated with a worse prognosis in these patients (38). The frequency of AKI in COVID-19 patients can vary and is related to worse outcomes in the disease population (39). Five of our patients (22%) had presented with AKI, which was in line with previous studies.
Cardiogenic shock is one of the important outcomes of COVID-19, which leads to a higher rate of mortality. A report from a Wuhan hospital on the first 138 patients showed that among ICU-admitted patients, 26% had increased D-dimer levels and 9% had shock (40).
Further studies comparing such outcomes between COVID-19 patients with or without DVT in a larger study population are warranted.
5.1. Conclusions
There was a higher mortality rate in COVID-19 patients who developed DVT during hospitalization. It could be proposed that for patients with severe COVID-19, higher coagulation parameters, and risk factors associated with thromboembolism, cardiac screening is important. Also, in patients who had preexistent cardiac disorder, echocardiographic evaluation at the time of admission could be useful.