1. Background
COVID-19 was first diagnosed in Wuhan, China, on December 31, 2019, and rapidly spread in Wuhan and other cities in China. As a result, the WHO declared it a global public health emergency (1). The latest WHO guidelines mention three primary ways of COVID-19 transmission: (1) transmission through respiratory droplets, especially during coughing and sneezing; (2) transmission through contact with contaminated surfaces, followed by touching the mouth, eyes, or nose with the hands; (3) transmission through aerosols.
Although COVID-19 has a relatively low mortality rate, it has a high potential for spread and transmission. Most patients fall within the age range of 30 to 79 years, although some studies report the age range as between 25 and 89 years. However, the number of infected children and infants remains very low (2). The viral load decreases over time in infected individuals, typically disappearing between the 9th and 14th day. Some studies, however, have detected the virus in the upper respiratory system using RT-PCR at lower levels, even after this period (2).
Coronavirus is an enveloped, single-stranded RNA virus that can infect a wide range of animals as well as humans. Early cases were diagnosed with pneumonia, but recent studies, particularly in children, have also reported gastrointestinal symptoms. In symptomatic patients, symptoms typically appear within a week, including persistent fever, cough, nasal congestion, fatigue, and shortness of breath (3). However, the full clinical manifestations of the disease remain unclear, as it ranges from mild cold-like symptoms to severe illness and death (4). Similar to other novel viral agents, there is no approved definitive treatment for COVID-19, and current treatment protocols in scientific literature are primarily supportive (4).
Thromboembolism refers to the formation of a clot inside blood vessels, which can travel through the vascular system and block blood flow in other parts of the body. In the mid-19th century, Rudolph Virchow proposed that thrombotic masses do not form directly in the pulmonary artery (PA) but instead result from the migration of clots from the peripheral venous system, causing secondary obstruction in PA branches (5). Larger blood clots originate from major veins, while smaller clots typically come from the crural or pelvic veins (6). Whole-body MR phlebography confirms the possibility of PA thrombosis without the presence of clots in the deep veins (7).
Histological evaluations of thrombosed PA branches have shown symptoms such as endothelial inflammation with edema, wall deposition, endothelial cell apoptosis, and infiltration of leukocytes, lymphocytes, and megakaryocytes in the vessel wall, which supports the diagnosis of thrombosis in the PA. During activation, the endothelium loses its anticoagulant properties and becomes a procoagulant phenotype. Thus, a disease characterized as thrombotic angiopathy can cause thrombosis in the PA (8).
Studies have reported an increased risk of arterial and venous thrombosis in patients with COVID-19. The findings of PA thromboses in the clear areas of patients' lungs, as seen in lung CT scans, suggest clot formation in these regions (9). The rising incidence of PA thrombosis in the context of COVID-19 contributes to the pathogenesis of respiratory failure, often necessitating mechanical ventilation. Thrombus-containing areas, in conjunction with permeable lung areas, suggest that clot formation may be linked to localized infection processes or anatomical inflammation (10).
Thrombogenicity in COVID-19 can be caused by several mechanisms: (1) modification of angiotensin II as a result of viral binding to the angiotensin-converting enzyme 2 (11); (2) alveolar damage and hypoxemia, which can further enhance vascular endothelial responses and promote thrombus formation (12).
Evidence supports the hypothesis of PA thrombi originating locally or in situ. Standard thromboprophylaxis at usual doses may be insufficient, and there is a consensus that enhanced preventive measures are necessary for COVID-19 patients. The severe course of COVID-19 is not only due to the inflammatory reaction in the lung parenchyma but also to thrombotic obstruction of small to medium pulmonary vessels, followed by lung parenchyma infarction, which is a key factor in the pathogenesis of acute respiratory distress syndrome (13).
2. Objectives
In this study, we examined the risk factors for pulmonary embolism in patients with COVID-19 hospitalized at Firoozabadi Hospital in 2020.
3. Methods
This retrospective cohort study was conducted to investigate the risk factors for pulmonary embolism in patients with COVID-19 hospitalized at Firozabadi Hospital from May to the end of December 2020. Demographic, clinical, and laboratory information of COVID-19 patients suspected of pulmonary thromboembolism (PTE) who underwent CT angiography were collected and recorded in a checklist. After extracting the required information, the data were entered into SPSS version 26 software and analyzed. The results of quantitative variables were expressed as mean and standard deviation (mean ± SD), while categorical qualitative variables were presented as percentages. The chi-square test was used to evaluate the relationship or difference between two qualitative variables, and Fisher's exact test was applied if the conditions for the chi-square test were not met. Additionally, the t-test was used under parametric conditions, and the Mann-Whitney U-test was applied under non-parametric conditions to compare a quantitative variable between two groups. For multi-group qualitative variables, the one-way ANOVA test was used in parametric conditions, and the Kruskal-Wallis X test was applied in non-parametric conditions. A significance level of 0.05 was considered.
The average values and frequency distribution of the demographic and clinical variables collected in the study are presented in the tables below (Tables 1 and 2). It is important to mention that pulmonary embolism was found to have a significant association with white blood cells (WBC) (P = 0.0001), lactate dehydrogenase (LDH) (P = 0.0001), D dimer (P = 0.0001), polymorphonuclear neutrophils (PMN) (P = 0.020), lymphocytes (P = 0.013), alkaline phosphatase (Alk.P) (P = 0.025), alanine aminotransferase (ALT) (P = 0.019), and aspartate aminotransferase (AST) (P = 0.017).
| Quantitative Variables | Pulmonary Thromboembolism | P-Value b | |
|---|---|---|---|
| Yes | No | ||
| Age (y) | 60.43 ± 15.02 | 58.22 ± 16.26 | 0.290 |
| WBC (× 109/L) | 11.15 ± 4.83 | 9.08 ± 4.82 | 0.0001 |
| Lymphocyte (× 109/L) | 11.59 ± 6.81 | 15.54 ± 11.23 | 0.013 |
| PMN (× 109/L) | 83.37 ± 8.67 | 79.30 ± 12.50 | 0.020 |
| RBC (× 1012/L) | 4.55 ± 0.70 | 4.59 ± 0.73 | 0.611 |
| Hemoglobin (mmol/L) | 13.16 ± 1.92 | 13.13 ± 2.17 | 0.862 |
| Platelets (× 109/L) | 224.98 ± 95.79 | 230.81 ± 104.91 | 0.750 |
| D Dimer (mg/L) | 7085.69 ± 5790.19 | 1282.54 ± 1182.74 | 0.0001 |
| CRP (mg/L) | 62.44 ± 34.95 | 55.28 ± 38.47 | 0.119 |
| ESR (mm/h) | 43.12 ± 24.36 | 39.40 ± 25.24 | 0.279 |
| LDH (IU/L) | 942.72 ± 571.30 | 705.46 ± 343.27 | 0.0001 |
| FBS (mg/dL) | 153.11 ± 63.31 | 159.87 ± 97.64 | 0.316 |
| BUN (mg/dL) | 22.14 ± 12.04 | 23.14 ± 14.75 | 0.744 |
| Creatinine (mg/dL) | 1.01 ± 0.35 | 1.11 ± 0.65 | 0.153 |
| Troponin (ng/L) | 31.46 ± 57.30 | 36.63 ± 81.38 | 0.221 |
| AST (IU/L) | 104.80 ± 287.63 | 63.88 ± 103.11 | 0.017 |
| ALT (IU/L) | 93.52 ± 242.67 | 50.65 ± 64.06 | 0.019 |
| ALP (IU/L) | 232.81 ± 82.64 | 224.88 ± 136.87 | 0.025 |
| Sodium (mmol/L) | 136.52 ± 4.01 | 135.83 ± 4.61 | 0.267 |
| Potassium (mmol/L) | 4.22 ± 0.61 | 4.30 ± 0.63 | 0.410 |
| PT (seconds) | 14.33 ± 2.74 | 14.06 ± 4.45 | 0.055 |
| PTT (seconds) | 43.47 ± 23.95 | 39.22 ± 19.01 | 0.241 |
| INR | 1.19 ± 0.41 | 1.19 ± 0.97 | 0.108 |
The Mean Status of the Studied Quantitative Variable a
| Quantitative Variables and Condition | Pulmonary Thromboembolism | P-Value b | |
|---|---|---|---|
| Yes | No | ||
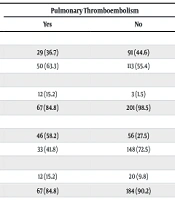
| Gender | 0.228 | ||
| Female | 29 (36.7) | 91 (44.6) | |
| Male | 50 (63.3) | 113 (55.4) | |
| Previous history of PTE | 0.0001 | ||
| Yes | 12 (15.2) | 3 (1.5) | |
| No | 67 (84.8) | 201 (98.5) | |
| Smoking | 0.0001 | ||
| Yes | 46 (58.2) | 56 (27.5) | |
| No | 33 (41.8) | 148 (72.5) | |
| Previous history of Asthma and COPD | 0.199 | ||
| Yes | 12 (15.2) | 20 (9.8) | |
| No | 67 (84.8) | 184 (90.2) | |
| Previous history of BP | 0.094 | ||
| Yes | 36 (45.6) | 71 (34.8) | |
| No | 43 (54.5) | 133 (65.2) | |
| Previous history of diabetes | 0.249 | ||
| Yes | 23 (29.1) | 46 (22.5) | |
| No | 56 (70.9) | 158 (77.5) | |
| Previous history of IHD | 0.543 | ||
| Yes | 16 (20.3) | 35 (17.2) | |
| No | 63 (79.7) | 169 (82.8) | |
Frequency Status of Qualitative Variable Under Study a
This study was conducted in accordance with the principles of the Declaration of Helsinki and the accepted regulations of the Iran University of Medical Sciences Ethical Committee (IR.IUMS.REC.1399.1239). Informed consent was obtained from all participants.
4. Results
Finally, the information of 283 COVID-19 patients referred to Firouzabadi Hospital in Tehran in 2020 was collected and recorded in a checklist. After gathering the necessary data, it was entered into SPSS software for analysis. The average age of the patients who participated in the study was 58.8 ± 15.9 years. Among the 283 participants, 162 patients (57.6%) were male, 79 patients (27.9%) had pulmonary embolism, 102 patients (36%) were smokers, and 107 patients (37.8%) had a history of hypertension.
5. Discussion
The COVID-19 pandemic has spread globally, affecting almost all countries around the world (14). In the early stages, symptoms include pneumonia, fever, muscle pain, fatigue, diarrhea, and loss of sense of smell and taste (2). Pulmonary embolism is the presence of a blood clot or, in some cases, fat in one of the pulmonary arteries or lung tissue. A blood clot or fat embolus travels through the bloodstream, passes through the heart, and lodges in one of the arteries that supply the lung tissue, leading to artery blockage. This results in decreased breathing ability and can cause lung tissue damage (5, 6). Recent studies have reported an increased likelihood of arterial and venous thrombosis in patients with COVID-19 (7-9).
In this study, 283 COVID-19 patients hospitalized at Firoozabadi Hospital from May to December 2020, who were suspected of having PTE and underwent CT angiography, were investigated to determine which variables might serve as risk factors for pulmonary embolism. The average age of the participating patients was 58.8 years, with the highest frequency observed in the age ranges of 60 - 65 years and 55 - 60 years. This suggests that individuals with PTE were generally older at the time of hospitalization. Additionally, they had a higher average age. This could indicate that as age increases, physical activity decreases, endothelial tissue dysfunction in the vessels increases, and consequently, the likelihood of clot formation and thrombosis also rises. It is also worth noting that there was no significant difference between the two groups, with and without thromboembolism (P = 0.290).
The gender distribution in this study was relatively balanced, with a slightly higher frequency of males. The male-to-female ratio was 1.35:1, and no significant difference was found between the genders in the two groups studied (P = 0.228). Regarding PTE, it was observed that only about a quarter (27.9%) of the individuals who underwent CT angiography had PA thromboembolism. Furthermore, only 5% of the studied subjects had a previous history of PTE. Among those without thromboembolism, only 1.5% had a prior history, while among those with thromboembolism, the rate was approximately ten times higher (15.2%). This highlights the significant role of a prior thromboembolism in contributing to the risk of new thromboembolism (P = 0.0001).
In terms of smoking, approximately one-third of the participants (36%) were smokers, with 27.5% among individuals without thromboembolism and 58.2% among those with thromboembolism, which is more than double. This demonstrates the effect of smoking on the incidence of thromboembolism (P = 0.0001).
Regarding underlying conditions such as asthma, chronic obstructive pulmonary disease (COPD), high blood pressure, diabetes, and ischemic heart disease, no significant evidence was found in this study to support their role as risk factors for thromboembolism (P > 0.05). However, studies with larger sample sizes and longer follow-up periods are needed to further investigate the relationship between these diseases and the incidence of lung thromboembolism.
White blood cell counts in individuals with PTE were significantly higher, falling within the leukocytosis range. Additionally, the differential CBC showed that the percentage of lymphocytes was significantly lower, while the percentage of polymorphonuclear neutrophils was significantly higher in those with thromboembolism. Therefore, it might be possible to consider that individuals with leukocytosis (characterized by a low lymphocyte percentage and a high PMN percentage) have a greater likelihood of developing thromboembolism, although the leukocytosis itself could be related to the COVID-19 infection.
As for D-dimer levels, it was observed that individuals with higher D-dimer levels clearly had a higher incidence of thromboembolism, which is a well-known association and requires no further explanation.
Regarding lactate dehydrogenase, it was found that individuals with thromboembolism had significantly higher LDH levels. An interesting finding in this laboratory evaluation was the presence of elevated liver function tests (LFT), with significantly higher values of AST, ALT, and Alk.P in the thromboembolism group compared to those without thromboembolism. This suggests that liver function evaluation is important and necessary for both the diagnosis and follow-up of thromboembolism cases.
In comparison with a study conducted by Cui L-y, which was a systematic review that examined 27 related articles out of 2210 articles, it can be noted that this study identified male gender, obesity, the need for mechanical ventilation, severity of pulmonary involvement, and elevated D-dimer and WBC levels as risk factors in COVID-19 patients with thromboembolism compared to those without thromboembolism. Similarly, in our study, high D-dimer and WBC levels were also found to be common risk factors, consistent with the findings of the aforementioned study. Regarding gender, although the male gender was generally more prevalent than the female gender in both the thromboembolism and non-thromboembolism groups (55.4% and 63.3%, respectively), this difference was not statistically significant (P = 0.228). Additionally, in both studies, patient age was not identified as a significant risk factor (15).
In comparison with a study conducted by Riyahi et al., both studies examined thromboembolism in COVID-19 patients using CT angiography. The time frame in their study was 5 months, while our study spanned 7 months. The sample size in their study was 413 patients, with thromboembolism occurring in 102 patients (24.6%), whereas our study, despite having a smaller sample size of 283 patients, had a similar thromboembolism incidence rate of 27.9%. The average age in their study was 60 years (with a standard deviation of 16 years), which was close to the average age in our study (58.8 years with a standard deviation of 15.9 years). The male gender frequency in their study was 55.7%, comparable to our study's 57.6%. An important point of comparison between the two studies is that both identified smoking, high D-dimer, and high LDH as risk factors for thromboembolism. Additionally, their study highlighted high ferritin as a risk factor, which was not investigated in our study (16).
5.1. Conclusions
Based on the results of this study, it can be concluded that the incidence of pulmonary embolism in hospitalized COVID-19 patients who underwent CT angiography with clinical suspicion of thrombosis was 27.9%. Additionally, factors such as smoking, a previous history of PTE, high WBC, elevated LFT, and high LDH are also considered risk factors for the occurrence of this disease.