1. Background
Acute coronary syndrome (ACS) encompasses a spectrum of medical conditions, including unstable angina, ST-segment elevation myocardial infarction (STEMI), and non-ST-segment elevation myocardial infarction (NSTEMI). Individuals diagnosed with ACS face an increased risk of recurrent cardiovascular events such as stroke and myocardial infarction, highlighting the importance of effective antiplatelet therapy in managing their condition (1-4).
Percutaneous coronary intervention (PCI) is a widely utilized treatment for ACS patients, aimed at restoring cardiac blood flow and preventing future cardiac events. Dual antiplatelet therapy (DAPT) post-PCI is essential to reduce stent thrombosis and recurrent ischemic complications. Notably, ticagrelor and clopidogrel are key DAPT inhibitors with distinct pharmacological profiles and clinical implications (5-7).
While clopidogrel has long been the standard antiplatelet therapy for patients undergoing PCI, the introduction of newer agents like ticagrelor has raised questions about their comparative effectiveness and safety (8, 9). Ticagrelor, a reversible P2Y12 receptor antagonist, has demonstrated superior efficacy in reducing cardiovascular mortality, myocardial infarction, and stroke in ACS patients compared to clopidogrel, as evidenced by the PLATO trial (10).
Adherence to antiplatelet therapy is pivotal in determining clinical outcomes, as non-adherence may lead to adverse events such as stent thrombosis and recurrent myocardial infarction (11). Of particular concern are bleeding events associated with antiplatelet agents (12, 13). Additionally, analyzing laboratory data, including lipid profiles and inflammation markers, offers valuable insights into the biological effects of these medications and their impact on cardiovascular risk (14). Evaluating the safety and efficacy of ticagrelor and clopidogrel provides essential guidance on optimal antiplatelet strategies for ACS patients undergoing PCI. These findings may inform personalized medicine approaches and contribute to enhancing patient care for this specific population (15, 16).
2. Objectives
This research project aimed to compare laboratory findings, adherence rates, and side effects following the administration of ticagrelor and clopidogrel to ACS patients post-PCI. This study holds promise in offering critical insights for clinical decision-making and advancing patient management for ACS individuals undergoing PCI. The results could significantly influence the development of evidence-based treatment strategies tailored to the unique requirements of this patient cohort. Through a real-world assessment of ticagrelor and clopidogrel utilization, this research aimed to illuminate their effectiveness and guide improved treatment modalities.
3. Methods
Here we conducted a comparative analysis to evaluate the effects of ticagrelor and clopidogrel in patients diagnosed with ACS following PCI. The research was performed on 200 ACS patients scheduled for PCI between 2022 and 2023 at hospitals affiliated with Shahid Beheshti University of Medical Sciences. Data collection involved gathering demographic and clinical information through interviews, questionnaires, and a review of patients' medical records. Baseline measurements of HDL, LDL, and CRP levels were recorded before treatment initiation to establish initial laboratory parameters.
The study employed specific inclusion criteria, requiring participants to have ACS and undergo angioplasty treatment, with an age range of 20 to 75 years. Exclusion criteria encompassed a history of inflammatory diseases, as well as blood, kidney, liver, and cancer diseases, along with regular use of anticoagulants. Random assignment of participants to receive either ticagrelor or clopidogrel was conducted, with adherence to standard dosing protocols for each medication to ensure uniform treatment. The randomization process was stratified by age, gender, and cardiac disease type to mitigate potential confounding factors. The research was conducted at Shahid Beheshti University of Medical Sciences in Tehran, Iran, with the approval of the Ethics Board (ethics code: IR.SBMU.MSP.REC.1402.561).
Patients were monitored for two months post-intervention, with repeated laboratory tests to assess HDL, LDL, and CRP levels. Primary outcomes focused on changes in these laboratory values, while secondary outcomes aimed to document adverse effects and evaluate medication compliance. Adverse events, including dyspnea, bleeding, and gastrointestinal symptoms, were systematically recorded for both groups throughout the study to evaluate medication safety profiles.
Medication adherence was evaluated through self-reported data from patients and pill counts during follow-up visits to ensure treatment regimen adherence. Descriptive statistics were used to summarize baseline participant characteristics, and comparative analyses of continuous outcomes were conducted using appropriate statistical tests, with adjustments made for multiple comparisons where necessary. The study adhered to the intention-to-treat principle, including all randomized patients in the analysis to maintain the integrity of the randomization process.
3.1. Statistical Analysis
Data analysis was performed using SPSS software version 21, with relationships between variables analyzed using the Pearson coefficient correlation test. Comparative analyses of parameters across different variables were conducted using tests such as the independent t-test. A significance level of P-value less than 0.05 was considered statistically significant for all analyses.
4. Results
The study included 200 patients with ACS who underwent PCI, consisting of 108 males (54%) and 92 females (46%), with an age range of 38 - 84 years and a mean age of 63.07 ± 12.54 years. The majority of patients presented with NSTEMI (76%), while the remaining had STEMI (24%). Among the STEMI subgroup, 27 patients (56.25%) received fibrinolytic therapy, and 21 patients (43.75%) underwent primary PCI. The prevalence of comorbidities associated with coronary involvement included diabetes in 24 individuals (12%), hypertension in 46 individuals (23%), and statin therapy for dyslipidemia in 36 individuals (18%). Following angioplasty, 63 patients (31.5%) received clopidogrel, while 137 patients (68.5%) received ticagrelor.
4.1. Comparison Between Clopidogrel and Ticagrelor Groups
The clopidogrel and ticagrelor groups did not show significant differences in terms of gender distribution, diabetes, hypertension, or the number of individuals receiving statins for dyslipidemia (P-value > 0.05). However, the average age in the clopidogrel group (65.68 ± 14.74) was significantly higher than that in the ticagrelor group (61.87 ± 19.11) (P-value < 0.001) ) (Table 1).
| Variables | Clopidogrel; (N = 63) | Ticagrelor; (N = 137) | Total; (n = 200) | P-Value |
|---|---|---|---|---|
| M/F | 108/92 (54)/(46) | 74/63 (54.01)/(45.99) | 34/29 (53.97)/(46.03) | 0.986 |
| Age | 12.54 ± 63.07 | 19.11 ± 61.87 | 14.74 ± 65.68 | < 0.001 |
| DM | 24 (12) | 16 (11.68) | 13 (20.63) | 0.683 |
| HTN | 46 (23) | 33 (24.09) | 13 (20.63) | 0.062 |
| Treated with statins due to dyslipidemia | 36 (18) | 27 (19.71) | 9 (14.29) | 0.788 |
a Values are expressed as No. (%), mean ± SD or unless otherwise indicated.
4.2. Complications
Shortness of breath occurred in 32 individuals (23.53%) in the ticagrelor group and 2 individuals (3.13%) in the clopidogrel group (P-value < 0.001). In the ticagrelor group, shortness of breath led to drug discontinuation in 4 patients (2.94%), whereas no patients in the clopidogrel group discontinued treatment due to shortness of breath. Gastrointestinal bleeding (melena) was observed in 3 patients (2.21%) in the ticagrelor group, with no instances in the clopidogrel group (P-value = 0.017). The occurrence of shortness of breath (P-value = 0.42) and bleeding (P-value = 0.32) did not show a significant association with the ACS presentation (NSTEMI or STEMI).
4.3. Laboratory Findings
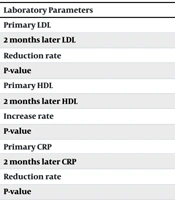
Despite no significant difference in initial LDL values between the clopidogrel and ticagrelor groups (P-value = 0.966), LDL levels were significantly lower after 2 months in the ticagrelor group (P-value = 0.002). However, the reduction in LDL did not significantly differ between the two groups (P-value = 0.11). A comparison of LDL values after 2 months with baseline values revealed a significant decrease overall and within each group (P-value < 0.001) (Table 2).
| Laboratory Parameters | Clopidogrel; (N = 63) | Ticagrelor; (N = 137) | Total; (n = 200) | P-Value |
|---|---|---|---|---|
| Primary LDL | 28.58 ± 136.22 | 28.29 ± 139.33 | 28.29 ± 129.59 | 0.966 |
| 2 months later LDL | 15.45 ± 59.50 | 14.53 ± 57.01 | 16.12 ± 64.78 | 0.002 |
| Reduction rate | 26.54 ± 76.72 | 26.80 ± 82.32 | 21.78 ± 64.81 | 0.110 |
| P-value | < 0.001 | < 0.001 | < 0.001 | |
| Primary HDL | 5.36 ± 33.78 | 5.66 ± 33.57 | 4.69 ± 34.22 | 0.210 |
| 2 months later HDL | 6.55 ± 40.75 | 6.38 ± 40.68 | 6.95 ± 40.89 | 0.132 |
| Increase rate | 4.45 ± 6.98 | 4.71 ± 7.12 | 3.86 ± 6.67 | 0.086 |
| P-value | < 0.001 | < 0.001 | < 0.001 | |
| Primary CRP | 5.18 ± 14.18 | 5.00 ± 14.11 | 5.60 ± 14.32 | 0.404 |
| 2 months later CRP | 1.72 ± 3.55 | 1.50 ± 3.09 | 1.77 ± 4.54 | 0.002 |
| Reduction rate | 4.80 ± 10.63 | 4.50 ± 11.02 | 5.33 ± 9.78 | 0.052 |
| P-value | < 0.001 | < 0.001 | < 0.001 |
a Values are expressed as mean ± SD.
Regarding HDL, a significant increase was observed after 2 months in both groups compared to baseline (P-value < 0.001), with no significant difference between the two groups in initial or 2-month values (P-value = 0.21 and P-value = 0.132) (Table 2).
Similarly, despite no significant difference in initial CRP values between the clopidogrel and ticagrelor groups (P-value = 0.404), CRP levels were significantly lower after 2 months in the ticagrelor group (P-value = 0.002). The reduction in CRP did not significantly differ between the two groups (P-value = 0.052). A comparison of CRP values after 2 months with baseline values demonstrated a significant decrease overall and within each group (P-value < 0.001) (Table 2).
5. Discussion
The study involved a cohort of 200 patients with ACS who underwent PCI, predominantly presenting with NSTEMI. Following angioplasty, patients received either clopidogrel or ticagrelor for antiplatelet therapy. Notably, no significant differences were observed between the two groups in terms of gender distribution, diabetes, hypertension, or statin use for dyslipidemia treatment.
Subsequent analysis indicated that ticagrelor exhibited superior efficacy in patients who underwent PCI compared to those planned for the procedure. However, the impact of ticagrelor varied based on ethnicity and geographical location (17, 18). A separate study evaluated the efficacy and safety of clopidogrel, ticagrelor, and prasugrel in patients with STEMI, demonstrating that ticagrelor and prasugrel were as effective as clopidogrel in reducing all-cause mortality and ischemic events without an increased risk of bleeding events (19).
Ticagrelor, a reversible P2Y12 receptor antagonist, has been associated with effects beyond platelet inhibition (20). While ticagrelor did not surpass clopidogrel in reducing major adverse cardiovascular events (MACE), it was linked to a higher bleeding risk. The additional therapeutic benefits of ticagrelor, particularly in lipid metabolism and inflammation reduction, warrant consideration by clinicians when choosing between ticagrelor and clopidogrel. Careful evaluation of these findings is essential to balance the potential benefits against bleeding risks (17, 20, 21).
Complications observed in the ticagrelor group, notably dyspnea and bleeding, align with previous research findings. Ticagrelor carries a higher bleeding risk compared to clopidogrel, even for minor bleeding events (17, 21). The safety concern regarding ticagrelor's elevated bleeding risk has been extensively studied, with meta-analyses indicating a significantly increased bleeding risk compared to clopidogrel (2).
The study's results align with the observed bleeding complications in the ticagrelor group, although the incidence of gastrointestinal bleeding was relatively low. Notably, breathlessness was reported more frequently in the ticagrelor group (22). However, breathlessness as a reason for discontinuing ticagrelor treatment was infrequent, suggesting that it may not commonly lead to treatment cessation. Interestingly, the ticagrelor group exhibited a significant reduction in LDL levels after two months, with no substantial difference in LDL reduction compared to the clopidogrel group. Although the initial LDL values did not significantly differ between the groups, this finding is noteworthy.
The decrease in CRP levels observed in the ticagrelor group after two months suggests potential anti-inflammatory effects of ticagrelor. Previous studies have indicated that ticagrelor may aid in reducing inflammation, potentially contributing to its cardiovascular benefits (23, 24).
5.1. Conclusions
In summary, the complications associated with ticagrelor, such as increased bleeding and dyspnea, are consistent with existing literature. However, the laboratory data indicating potential benefits of ticagrelor, such as reducing LDL and CRP levels, suggest additional therapeutic effects that warrant further investigation. Clinicians should carefully assess the risks and benefits when considering ticagrelor for ACS patients undergoing PCI.