1. Background
The adoption of the transradial approach (TRA) for coronary angiography and percutaneous coronary intervention (PCI) has significantly increased globally over the past decade (1). Although TRA may present greater complexities than the transfemoral approach, its numerous advantages, including a decreased incidence of complications and adverse outcomes, have made it increasingly favorable (2-7). Transradial approach is recognized as a safer alternative, evidenced by numerous studies demonstrating lower rates of complications compared to the femoral approach (4, 6).
Coronary angiography is widely regarded as the gold standard for diagnosing coronary artery disease, serving both diagnostic and therapeutic purposes (8, 9). However, the procedure's invasive nature can induce considerable stress and anxiety in patients. Complications associated with angiography, such as hematoma, bleeding, thrombosis, and arterial emboli, are often linked to trauma to the femoral artery (10). Moreover, the transfemoral approach necessitates that patients remain in a supine position for extended periods, which can lead to discomfort and various side effects, including groin and leg pain, back pain, urinary difficulties, and nausea. This discomfort, particularly from back pain, has prompted efforts to enhance patient care protocols (11).
Despite the transfemoral approach's association with shorter hospital stays and lower incidences of bleeding and hematoma, it carries risks, including radial artery spasm (RAS), which can affect up to 30% of patients undergoing TRA (12). Research indicates that moderate to severe pain during arterial cannulation significantly increases the likelihood of RAS (12). The radial artery's high concentration of adrenoceptors renders it susceptible to contraction induced by catecholamines (13). Consequently, minimizing pain during TRA may have a beneficial impact on reducing the incidence of RAS (14).
The eutectic mixture of local anesthetics (EMLA), comprising lidocaine and prilocaine, has shown efficacy as a local anesthetic in various procedures, including arterial cannulation, and has been associated with shorter cannulation times and improved success rates. However, its slower onset of action compared to direct lidocaine injections may limit its clinical application (15). Current evidence comparing the efficacy of EMLA cream with local lidocaine injection is limited and, at times, contradictory, lacking clarity regarding both success rates and associated side effects.
2. Objectives
Given these considerations, the present study aims to evaluate the efficacy of lidocaine cream in conjunction with subcutaneous injection for pain reduction in patients undergoing TRA, as well as to determine whether the use of lidocaine cream may enhance cannulation success rates and reduce the incidence of RAS.
3. Methods
3.1. Study Design and Setting
This study was a single-blinded clinical trial conducted from 2022 to 2023 at Shahid Modarres Hospital in Tehran, Iran. The sample size was determined using Cochran's formula (16), with the population size estimated at 236 individuals. Given the two-domain nature of the study, which compares two treatment methods, the parameters used for sample size calculation included (d = 0.1), (Z = 1.96), (P = 0.5), and (q = 0.5). This calculation initially suggested a sample size of 68 participants. To accommodate potential dropouts, a total of 80 patients were recruited and randomly allocated into two groups of 40. The study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (approval ID: IR.SBMU.RETECH.REC.1400.1048).
3.2. Participant Selection
The participant selection process involved recruiting individuals over the age of 18 who were candidates for radial artery angiography. Participants were screened according to specific inclusion criteria, which required them to have no arteriopathy in the upper limb, a normal Allen test, no history of Raynaud's disease, no Q-wave myocardial infarction in the week preceding the study, no known allergies to contrast material, and the ability to provide informed consent. Conversely, individuals were excluded from the study if they had used any analgesics or had a documented allergy to lidocaine. This rigorous selection process aimed to ensure a homogeneous study population and the validity of the study outcomes.
3.3. Randomization and Blinding
Participants were randomly assigned to either the lidocaine-P group (receiving lidocaine cream and subcutaneous injection of lidocaine) or the control group (receiving placebo cream with subcutaneous lidocaine). To maintain blinding, participants were unaware of the cream contents (lidocaine-P or placebo). Informed consent was obtained from all participants after explaining the study's purpose, methodology, potential risks, and complications.
3.4. Anesthesia Administration
Lidocaine-P cream (Tehran Shimi Pharmaceutical Company) was applied two hours prior to the procedure, while zinc oxide cream served as the placebo.
3.5. Pre-procedure Assessment
To ensure adequate ulnar artery health, a peripheral vein in the left upper limb was accessed, and the Allen test was performed prior to angiography.
3.6. Angiography Procedure
Following radial angiography, the site where the TR band (a device designed to exert pressure on the radial artery) was applied was assessed for hematoma and bleeding, and the distal pulse at the angiography site was evaluated. For diagnostic angiography, air evacuation from the TR band began 30 minutes post-procedure, while in cases of angioplasty, this commenced after 60 minutes. Nurses monitored the amount of air in the TR band, evacuating 2 mL of air every half hour. In instances of bleeding during air evacuation, the equivalent air volume was reinflated, followed by a reassessment for bleeding. In cases of hematoma, the site was marked, and if there was progression, a blood pressure cuff was applied proximally and inflated to the mean arterial pressure (MAP). For diagnostic cases, patients were discharged three hours after TR band removal. In cases of significant pain or swelling at the angiography site, a Doppler ultrasound was ordered to evaluate for pseudoaneurysm.
3.7. Data Collection and Assessment
Pain intensity was evaluated using the Visual Analog Scale (VAS) (17). Spasm occurrence was assessed by the attending physician, while signs of vasovagal syndrome—including blood pressure, heart rate, nausea, and sweating—were measured through clinical judgment. Demographic data (age, gender, etc.) were recorded using a pre-designed questionnaire. A separate checklist was employed to classify complications and underlying health conditions. Following the angiography process, all questionnaires and checklists were reviewed and completed.
3.8. Statistical Analysis
Data were analyzed using SPSS version 23. Initial analyses were descriptive, employing tables, graphs, and indices of central tendency and dispersion. Subsequently, inferential analyses were conducted using Student’s t-test for independent groups and chi-square tests to explore hypotheses regarding differences between groups. Ethical approval for the study was obtained from the relevant institutional review board, ensuring adherence to standards for the ethical treatment of participants.
4. Results
In this study, a total of 80 patients undergoing radial angiography were included, divided into two groups of 40 each: One group receiving lidocaine-P cream and the other receiving a placebo cream. The mean age of the participants was 58.10 years, with a standard deviation of 10.88. The youngest participant was 32 years old, and the oldest was 88 years old. Specifically, the mean age of the control group was 58.25 years (SD = 11.15), while the experimental group (lidocaine-P) had a mean age of 57.95 years (SD = 10.75).
In terms of gender distribution, the experimental group comprised 16 females and 24 males, while the control group also consisted of 16 females and 24 males, indicating an equal gender distribution in both groups.
Before conducting statistical analyses and hypothesis testing, the demographic and clinical characteristics were assessed for equivalence between the two groups. The distribution of the groups concerning other variables was evaluated using chi-square tests or Fisher's exact tests, depending on the data type. The analysis revealed that the differences between the lidocaine-P and placebo groups were not statistically significant, suggesting that the two groups were comparable in demographic and clinical characteristics (Table 1).
| Variables | Control Group (Placebo) | Experimental Group (Lidocaine-P) | P-Value |
|---|---|---|---|
| Gender | 1.00 | ||
| Male | 24 (60) | 24 (60) | |
| Female | 16 (40) | 16 (40) | |
| Age | 1.00 | ||
| 29 - 40 years | 3 (7.5) | 3 (7.5) | |
| 40 - 50 years | 5 (12.5) | 5 (12.5) | |
| Over 50 years | 32 (80) | 32 (80) |
Distribution of Patients Undergoing Angiography Receiving Lidocaine-P or Placebo Based on the Evaluated Variables
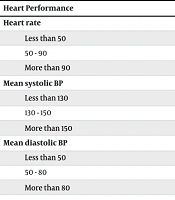
Additionally, prior to angiography, the systolic blood pressure, diastolic blood pressure, and heart rate of the participants were recorded (Table 2). The differences in mean vital signs were analyzed using chi-square tests or Fisher's exact tests, as appropriate for the data. Statistical analysis indicated no significant difference in systolic blood pressure (P = 0.132), diastolic blood pressure (P = 0.565), and heart rate (P = 0.372) between the two groups.
| Heart Performance | Control Group (Placebo) | Experimental Group (Lidocaine-P) | P-Value |
|---|---|---|---|
| Heart rate | 0.372 | ||
| Less than 50 | 0 (0) | 0 (0) | |
| 50 - 90 | 36 (90) | 31 (77.5) | |
| More than 90 | 4 (10) | 9 (22.5) | |
| Mean systolic BP | 0.132 | ||
| Less than 130 | 25 (62.5) | 16 (40) | |
| 130 - 150 | 10 (25) | 16 (40) | |
| More than 150 | 5 (12.5) | 8 (20) | |
| Mean diastolic BP | 0.565 | ||
| Less than 50 | 1 (2.5) | 0 (0) | |
| 50 - 80 | 36 (90) | 36 (90) | |
| More than 80 | 3 (7.5) | 4 (10) |
Heart Performance Metrics of Patients Undergoing Angiography Receiving Lidocaine-P or Placebo Based on Evaluated Variables
As shown in Table 3, following radial angiography and data collection, it was determined that six individuals in the control group experienced vasovagal syndrome, whereas only one individual in the experimental group was affected. Statistical testing indicated that the occurrence of vasovagal syndrome was significantly higher in the control group (P = 0.011).
| Vasovagal Syndrome | Control Group (Placebo) | Experimental Group (Lidocaine-P) | P-Value |
|---|---|---|---|
| Yes | 6 (15) | 1 (2.5) | 0.011 |
| No | 34 (85) | 39 (97.5) |
Prevalence of Vasovagal Syndrome in Patients Undergoing Angiography Receiving Lidocaine-P or Placebo Based on Evaluated Variables
Furthermore, the findings indicated that none of the participants in the experimental group reported experiencing pain, while seven participants in the control group reported pain (Table 4). Statistical analysis confirmed that the difference in the number of individuals reporting pain between the two groups was significant (P = 0.006).
| Pain Presence | Control Group (Placebo) | Experimental Group (Lidocaine-P) | P-Value |
|---|---|---|---|
| Yes | 7 (17.5) | 0 (0) | 0.006 |
| No | 33 (82.5) | 40 (100) |
Prevalence of Pain Among Patients Undergoing Angiography Receiving Lidocaine-P or Placebo Based on Evaluated Variables
The study also revealed that five patients in the control group experienced spasms, with no reports of spasms in the experimental group. Statistical results showed that the incidence of spasms was significantly higher in the control group (P = 0.021) (Table 5).
| Spasm Presence | Control Group (Placebo) | Experimental Group (Lidocaine-P) | P-Value |
|---|---|---|---|
| Yes | 5 (12.5) | 0 (0) | 0.021 |
| No | 35 (87.5) | 40 (100) |
Prevalence of Spasms in Patients Undergoing Angiography Receiving Lidocaine-P or Placebo Based on Evaluated Variables
5. Discussion
Pain management is a paramount concern in the emergency department, and patient sedation is a crucial aspect of this endeavor (18, 19). The alleviation of pain and its treatment are significant health concerns, often hampered by the safety risks associated with systemic medications. In Europe, it is estimated that 25 - 30% of the population suffers from chronic pain. Despite the availability of recommended treatments, more than 60% of patients with chronic pain fail to exhibit improvement or respond poorly, frequently experiencing adverse events (AE) (20). The literature recommends the use of topical agents as first- or second-line treatment options for pain management (20, 21). The objective of this study was to investigate the efficacy of lidocaine cream in reducing pain, spasm, and vasovagal syndrome. The findings of this study demonstrate that the use of this cream can be an effective adjunct in mitigating the pain associated with angiography. Chronic pain is a debilitating condition affecting approximately 5 million adults in the U.S. each year, resulting in over $2 billion in lost medical costs and wages. Defined as pain lasting beyond six months, it is recognized as a major public health issue and termed a "silent epidemic." Despite improvements in pain management, the prevalence of chronic pain continues to rise. A 2021 National Health Interview Survey found that around 1.5 million adults experienced daily pain for six months, with 2 million reporting significant or severe pain. In recognition of its impact, the American pain community designated pain as the fifth vital sign in 2021 (22). Lidocaine is the most effective local anesthetic belonging to the amide group. It is a moderate topical anesthetic known for its rapid onset of action. Beyond its role in pain management, lidocaine is valuable for controlling neuropathic pain and is also used to treat both acute and chronic pain, as well as to manage arrhythmias. Additionally, it is utilized in addressing chronic pain associated with neurological disorders, including myofascial pain, stroke, and neurogenic pain (18, 23, 24). The findings of this study indicate that the cream tested may effectively reduce spasms associated with radial angiography. Spasms are broadly defined as sensory motor disorders resulting from lesions in upper motor neurons. Symptoms can include increased muscle tone, contractions, exaggerated reflexes (clonus), and limited joint mobility. The severity of spasms can vary, ranging from mild stiffness to intense, painful contractions, typically occurring in the legs and arms (25). In addition to the immediate symptoms, spastic musculoskeletal issues may arise, such as decreased muscle movement and stability. Side effects of spasms can include functional impairment due to muscle stiffness, uncontrollable and often painful contractions, deformities of muscles and joints, abnormal muscle growth, and inhibited protein synthesis in muscle cells (26). There are several effective treatments available for managing and treating muscle spasms (27). The primary therapeutic goals in the context of spasms include alleviating symptoms, reducing pain and excessive muscle contractions, and improving mobility and overall quality of life. One of the most commonly used therapies for muscle spasms involves the administration of medications such as lidocaine. Most pharmacological interventions aim to reduce the release of excitatory neurotransmitters (e.g., glutamate and monoamines) or enhance the release of inhibitory neurotransmitters (such as glycine and others) to diminish reflex activity (28). Vasovagal syndrome, often referred to as neurotransmitter syndrome or neurological syndrome, can occur frequently as a result of the activation of cardiac nerve pathways during medical procedures, posing challenges for healthcare providers (29). The annual prevalence of vasovagal syndrome is estimated to range from 0.5% to 2.5% per person (30). Specifically, during cardiovascular interventions, the prevalence is similarly cited between 0.5% and 2.5%, while for colonoscopy procedures, it is also within the same range. The associated mortality rates for vasovagal syndrome are reported to be between 0.5% and 2.5%. Findings from this study suggest that the use of the cream can significantly reduce the incidence of vasovagal syndrome during radial angiography. Factors such as pain, tissue injury, and strong emotional responses can exacerbate this condition. In patients with mild pathology, symptoms may only present as pre-syncopal or fainting episodes (including dizziness, mental confusion, weakness, and diaphoresis) without loss of consciousness. Most patients do not experience permanent loss of consciousness. While the condition is generally benign, some reports indicate that a more severe form can lead to significant long-term cardiac issues, often precipitated by surgical interventions. If prolonged, these episodes may lead to asystole and, in extreme cases, sudden cardiac arrest. It has been suggested that topical anesthetics, such as lidocaine and its derivatives, may help alleviate pain or prevent the onset of vasovagal syndrome (31).
5.1. Conclusions
The findings of this study suggest that the application of lidocaine cream can effectively reduce pain, spasms, and the incidence of vasovagal syndromes associated with radial angiography, thereby minimizing potential complications. Given that radial angiography offers several advantages over femoral angiography, including faster recovery times and less invasive procedures, the use of this cream may enhance patient comfort and safety. By alleviating associated symptoms, the lidocaine cream could encourage both patients and physicians to opt for radial angiography more frequently as a preferred method for vascular interventions.